
January 24 - 26
Visit us at Booth #414 to Discover
Artivion’s EDGE
Jump to a section to learn more:

Dr. Wilson Szeto
University of Pennsylvania, USA

Dr. William Brinkman
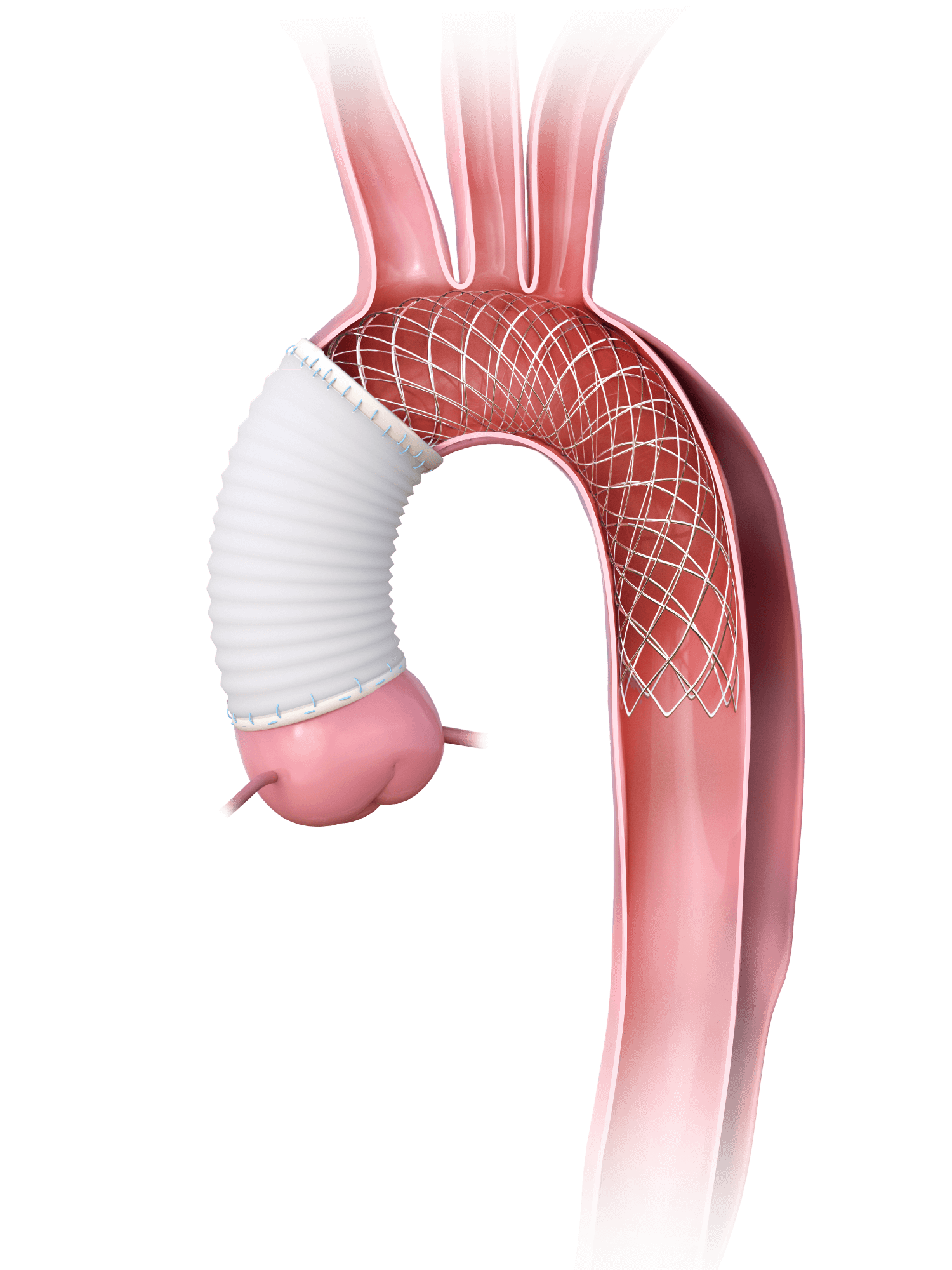
Introduction to AMDS and Results from the PERSEVERE US IDE Study
Baylor Scott and White Health, USA

Prof. Joerg Kempfert
History of AMDS: How it Works and World-wide Experience
German Heart Center Berlin, Germany

Dr. Fernando Fleischman
Best Practices for Success: Patient Selection and Implantation Techniques
University of Southern California, USA
Booth Talks
Visit us at Booth 414 to learn about the compelling clinical evidence behind our solutions
Optimizing Mitral Valve Replacement for Patients With Longer Life Expectancy
Friday, January 24th
9:15 AM - 9:30 AM
Add event to calendar

Division Head and Minimally Invasive Cardiac Surgery Chair, Division of Cardiac Surgery
University of Ottawa Heart Institute, Canada
Podium Presentations
Late-Breaker Presentation:
Key Sessions of Interest:
Session Title: Arch Disease: Be Bold?
Topic: Open Arch Bare Metal Stenting in Type A Aortic Dissections
Thursday, January 23rd
3:15 - 3:22 PM
Chief, Division of Cardiovascular Surgery
University of Pennsylvania Medicine, USA
Session Title: Lifetime Strategy for Thoracic Aortic Disease
Topic: How I Do It: Open Arch Bare Stenting in ATAAD
Friday, January 24th
10:23 - 10:31 AM
Chief, Division of Cardiovascular Surgery
University of Pennsylvania Medicine, USA
Poster Presentations:
Mechanical vs Biological Valves for Modified Bentall Procedure; 10-Year Outcomes from a High-Volume Aortic Center

Cardiac Surgery Department
IRCCS-Azienda Ospedaliero
Universitaria di Bologna, Italy
The North American Ross
Consortium: 30-Day Retrospective
Outcomes

General Surgery Resident
Baylor University Medical Center, USA
The industry symposium and booth talks will be held in conjunction with the STS Annual Meeting. It is not part of the official STS scientific program. Continuing Medical Education (CME) credit for this activity is not offered by STS.
AMDS is RX Only: Humanitarian Use Device. Authorized by Federal Law for use in the treatment of Acute DeBakey Type I Aortic Dissection. The effectiveness of this device for this use has not been demonstrated. NEXUS is an investigative device limited by Federal (or United States) law to Investigation use only within the United States. All products and Indications are not available / approved in all markets.






