
AMDS™
HYBRID PROSTHESIS
Elevate the Standard.
The world’s first device designed specifically to address the unique challenges of acute type A aortic dissection, specifically DeBakey Type I (ADTI) with malperfusion.
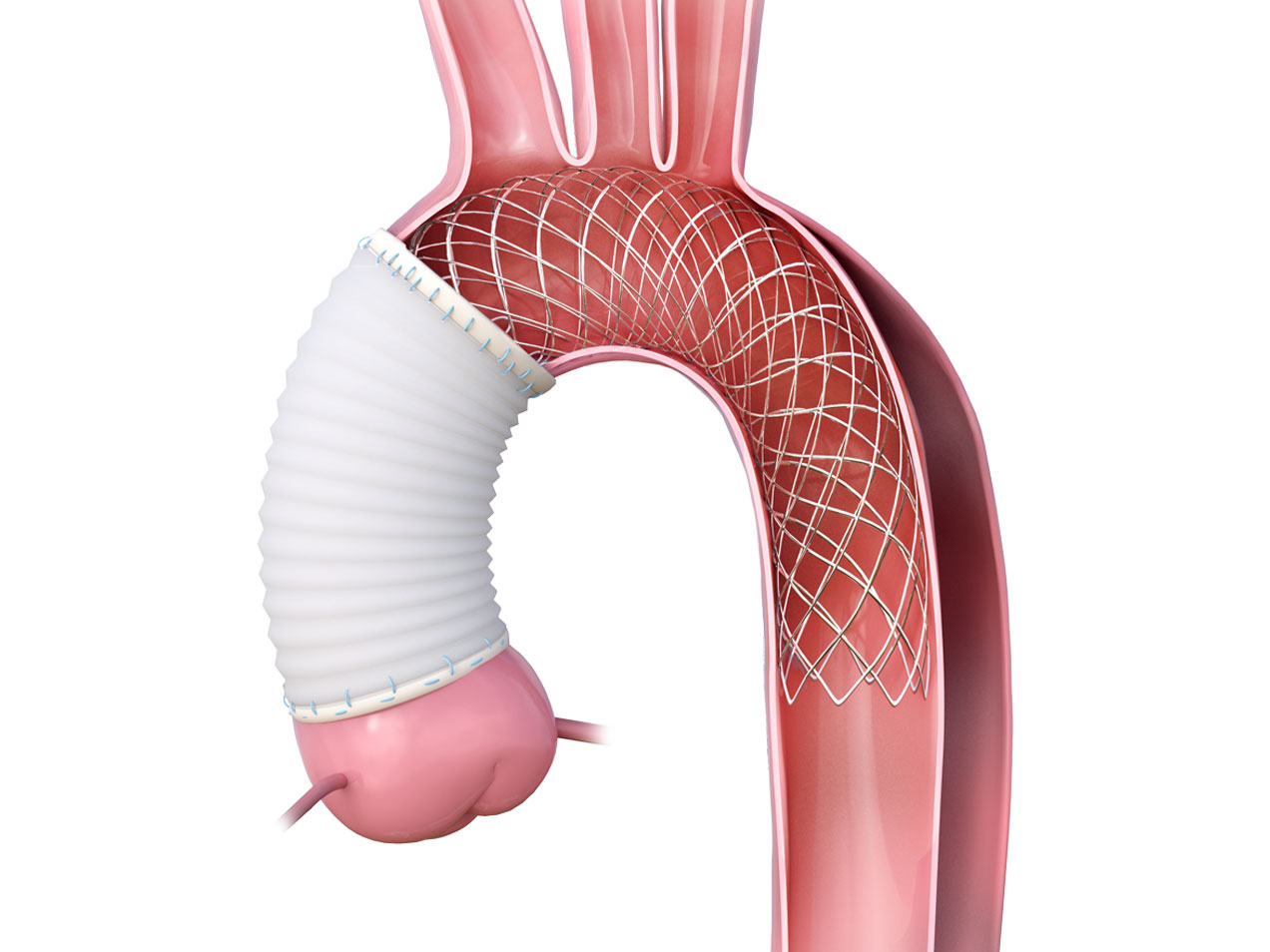
Product Highlights
-
Reduction in Major Adverse Events (MAE’s)1-7
-
Prevention of DANE6-9
-
Resolution of Malperfusion9
-
Promotes Positive Aortic Remodeling6-8
-
Ease of Use6
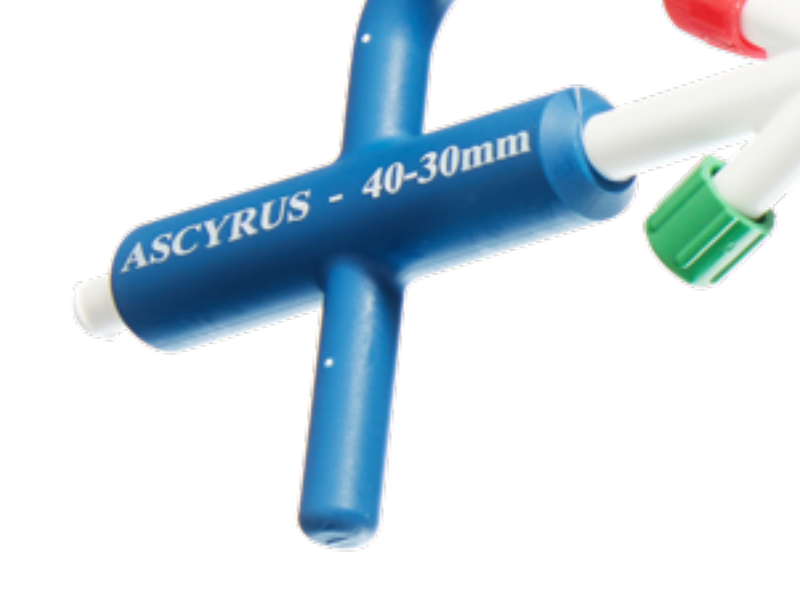
Product Overview
1. PTFE FELT CUFF

Component made of a PTFE felt tube is used to buttress and strengthen the aortic tissue in preparation to perform the conventional polyester graft to aorta anastomosis.
2. UNCOVERED NITINOL WIRE BRAIDED STENT
Expands and supports the true lumen across the aortic arch and descending aorta thereby stabilizing the aortic wall and promoting remodeling.
3. STENT SUPPORTED CUFF

The stent supported cuff of the AMDS and expansion of the AMDS from the arch distally, elevates and supports the intimal flap, reducing the tension on the suture line, avoiding the formation of DANEs in the friable anastomosis.
4. DESIGNED TO AVOID dSINE
When deployed in vessel, the distal ends of the stent are designed to point away from the aortic wall to avoid Distal Stent Graft-Induced New Entrys (dSINE).7,8
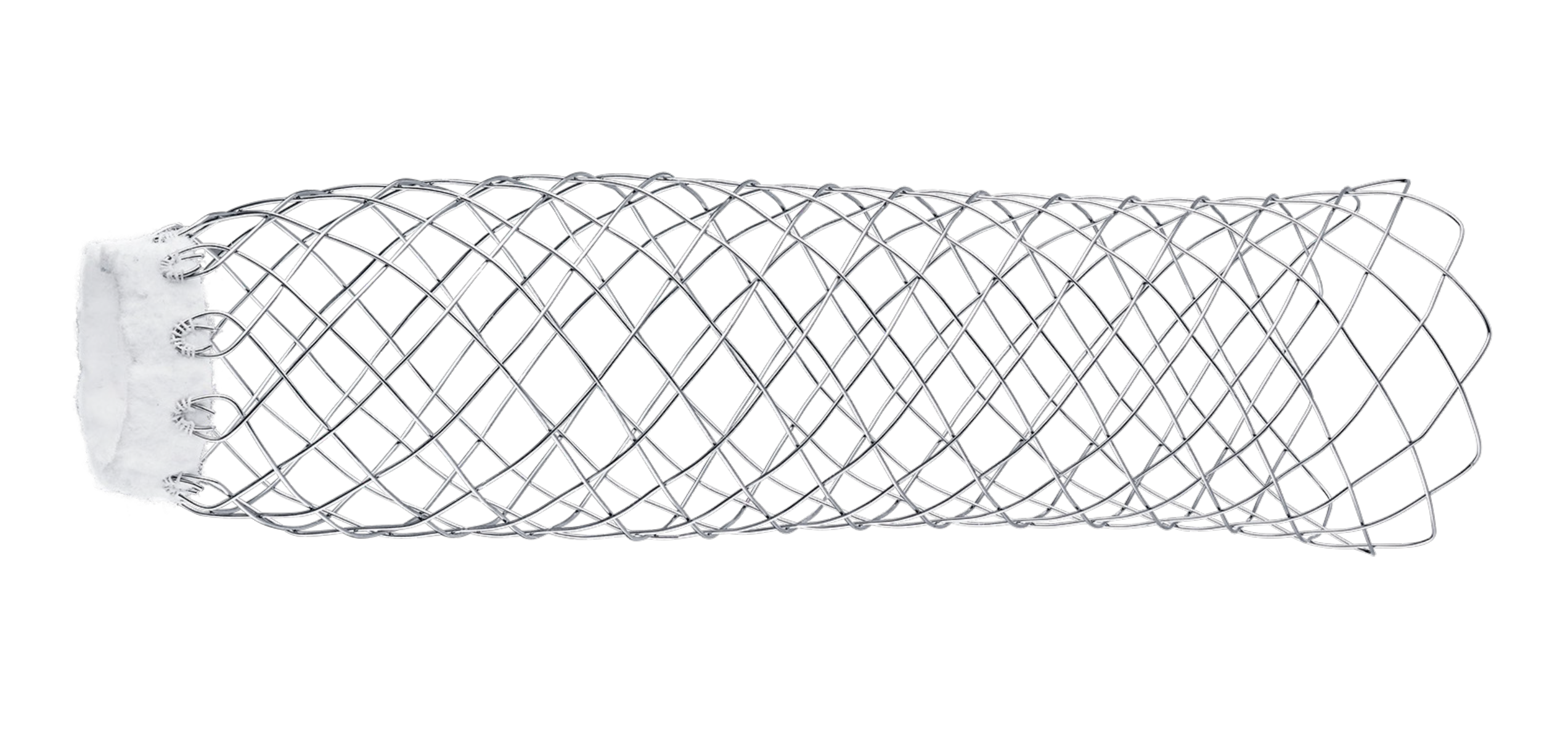
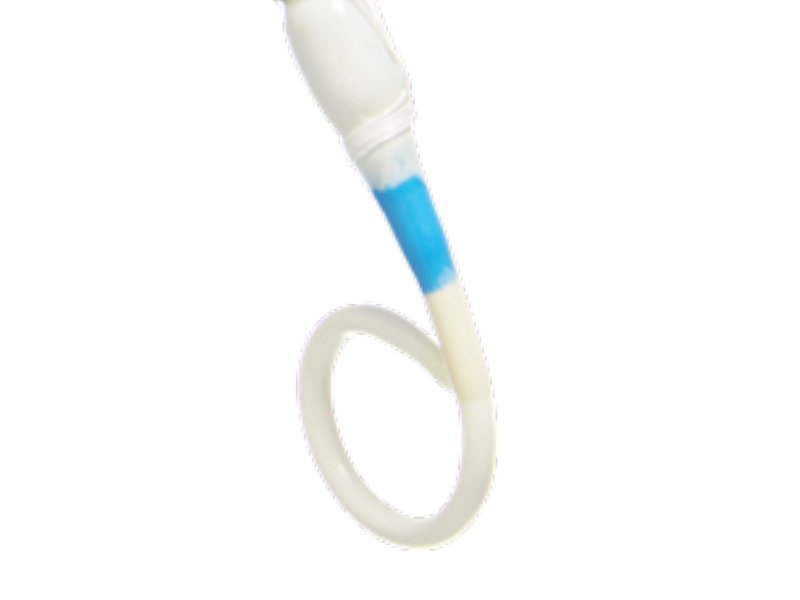
1. RED CAP
Not functional.

2. GUIDEWIRE (GW) EXIT
Compatible with 0.035” GW (optional).
3. HANDLE
Ergonomic handle for easy grip during delivery.

4. GREEN CAP
To deploy/release the suture constraining the stent.

5. PROTECTIVE SHEATH
For simple and atraumatic introduction to the transected aorta (6 cm long).

6. LOADED STENT
Flexible catheter shaft for simple tracking of system and atraumatic to vessel.

7. PIGTAIL SHAPED TIP
Pigtail tip reduces risk of device tracking into FL via a entry tear/fenestration.

“Knowing that you’re giving people surgery that is not having any additional complexity but with a better outcome is very reassuring.”
“A new tool in the toolbox for surgeons to do a better operation at the time of an index operation for a DeBakey I.”
Clinical Evidence
PERSEVERE US IDE Study Early and Midterm Results:
A Landmark Prospective, Multi-Center Study to Evaluate the Safety and Effectiveness of AMDS™ in the Treatment of Acute DeBakey Type I Dissection (ADTI) with Malpersusion.

Key 30-day PERSEVERE US IDE Results
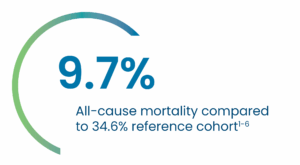
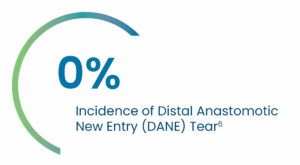
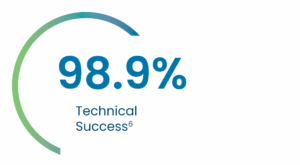
Average AMDS implant time* = 15 minutes6
Key 1-year PERSEVERE US IDE RESULTS
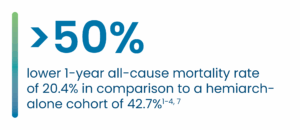

AMDS promotes positive aortic remodeling by maintaining a stable total aortic diameter while sustaining the true lumen and continuing to thrombose the false lumen.7
Additional Resources
Explore additional resources for AMDS below. For further information or to contact a sales associate in your area, contact us.
- Zindovic I, G. T., Ahlsson A, Fuglsang S, Gunn J, Hansson EC, Hjortdal V, Jarvela K, Jeppsson A, et al. . (2019). Malperfusion in acute type A aortic dissection: an update from the Nordic Consortium for Acute Type A Aortic Dissection. J Thorac Cardiovasc Surg.,157, 1324-1333.
- Pacini D, L. A., Belotti LM, Fortuna D, Gabbieri D, Zussa C, Contini A, Di Bartolomeo R, et al. (2013). Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg, 43, 820-826.
- Geirsson A, S. W., Pochettino A, McGarvey ML, Keane MG, Woo YJ, Augoustides JG, Bavaria JE. (2007). Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Su, 32, 255-262.
- Girdauskas E, K. T., Borger MA, Falk V, Mohr FW. (2009). Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg, 138, 1363-1369.
- Bossone E, R. V., Nienaber CA, Trimarchi S, Ballotta A, Cooper JV, Smith DE, Eagle KA, Mehta RH. (2002). Usefulness of Pulse Deficit to Predict In-Hospital Complications and Mortality in Patients With Acute Type A Aortic Dissection. Am J Cardiol, 89, 851-855.
- Szeto WY, Fukuhara S, Fleischman F, Sultan I, Brinkman W, Arnaoutakis G, Takayama H, Eudailey K, Brinster D, Jassar A, DeRose J, Brown C, Farrington W, Moon MC. A novel hybrid prosthesis for open repair of acute DeBakey type I dissection with malperfusion: Early results from the PERSEVERE trial. J Thorac Cardiovasc Surg. 2024 Aug 6:S0022-5223(24)00677-9.
- Adjudicated data as presented at the 61st STS Annual Meeting by Dr. S. Fukuhara on behalf of corresponding authors, One-Year Results of a Novel Aortic Arch Hybrid Prosthesis for Open Repair of Acute DeBakey Type I Dissection with Malperfusion in the PERSEVERE Study / Friday, January 24, 2025 / 10:00 am – 10:15 am.
- Bozso SJ, N. J., Chu MWA, Kiaii B, El-Hamamsy I, Ouzounian M, Forcillo J, Kempfert J, Stark C, Moon MC. (2022). 3-Year Outcomes of the Dissected Aorta Repair Through Stent Implantation Trial. J Thorac Cardiovasc Surg.
- Bozso, Sabin J. et al. Single-Stage Management of Dynamic Malperfusion Using a Novel Arch Remodeling Hybrid Graft. The Annals of Thoracic Surgery, Volume 108, Issue 6, 1768 – 1775. DOI: https://doi.org/10.1016/j.athoracsur.2019.04.121
RX Only: Humanitarian Use Device. Authorized by Federal Law for use in the treatment of acute DeBakey Type I Aortic Dissection with malperfusion. The effectiveness of this device for this use has not been demonstrated. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc. Jotec GmbH and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. © 2024 Artivion, Inc. All rights reserved. MWENG0019.002. (2025-09).
| Artivion, Inc. 1655 Roberts Blvd., NW Kennesaw, GA 30144 USA |

