VEITHsymposium 2022
November 15 – November 19, 2022
New York Hilton Midtown Hotel | Booth 512
The 49th VEITHsymposium is quickly approaching, and we’re excited to attend this meeting live in-person at New York Hilton Midtown Hotel. Remember you will need to be registered with VEITHsymposium to attend any of the scientific sessions. Click here to view the complete list of scientific sessions happening this year at VEITHsymposium. We look forward to connecting with you. Visit us at Booth 512!
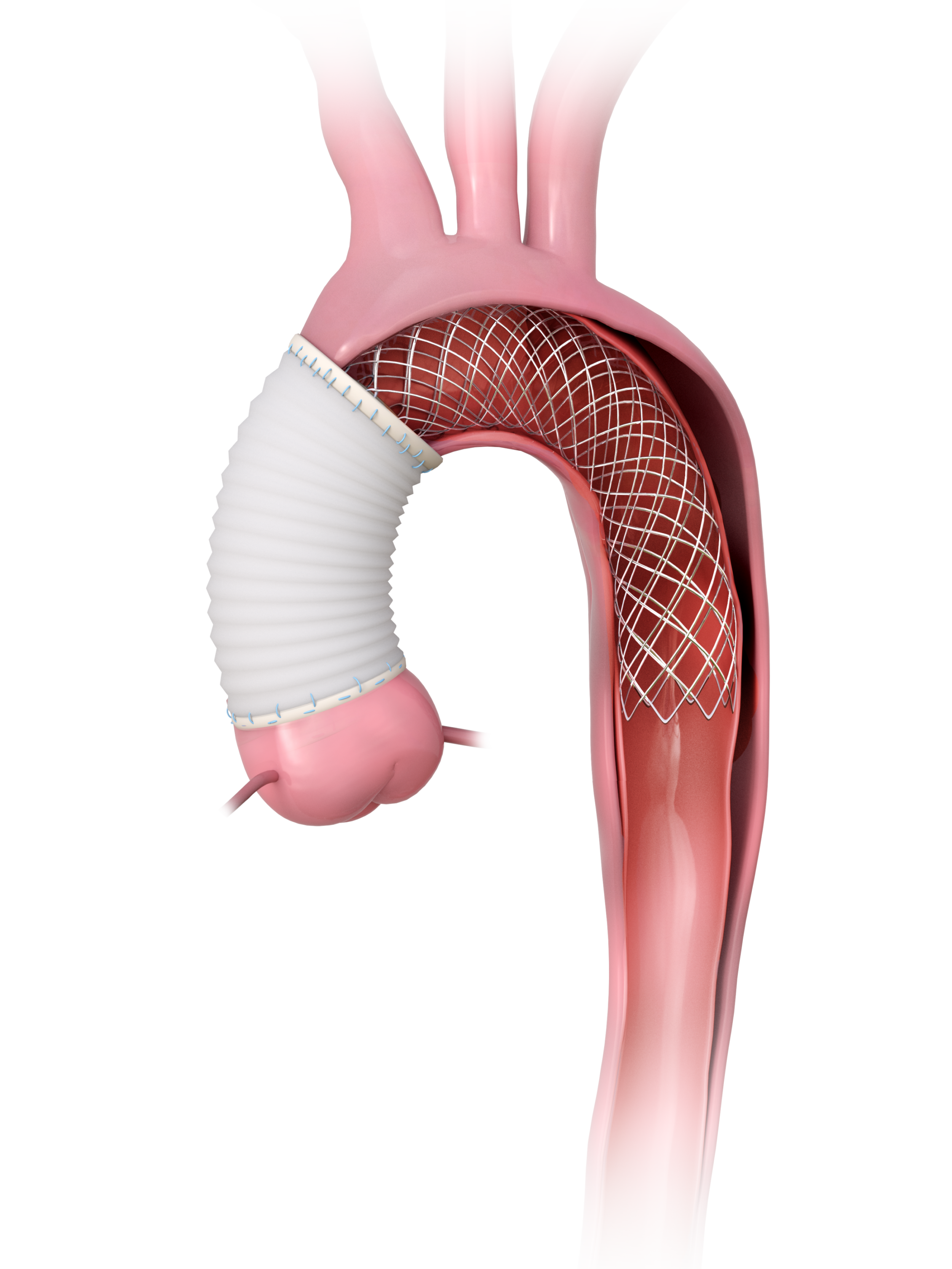
Comparison of Early and Midterm Results with 2-Branched Endograft (Relay – Terumo Aortic) and Single Branched Device (Nexus – Endospan) for Treatment of Zone 0 Arch Lesions.
Presented by: Tori Kuratani, MD, PhD
Abstract Presentation:
Inner Branched Complex Aortic Repair Outcomes From A National Multicenter Registry Using The E-Xtra Design Platform (The CELER Study)
Presented by: Gioele Simonte, MD, PhD
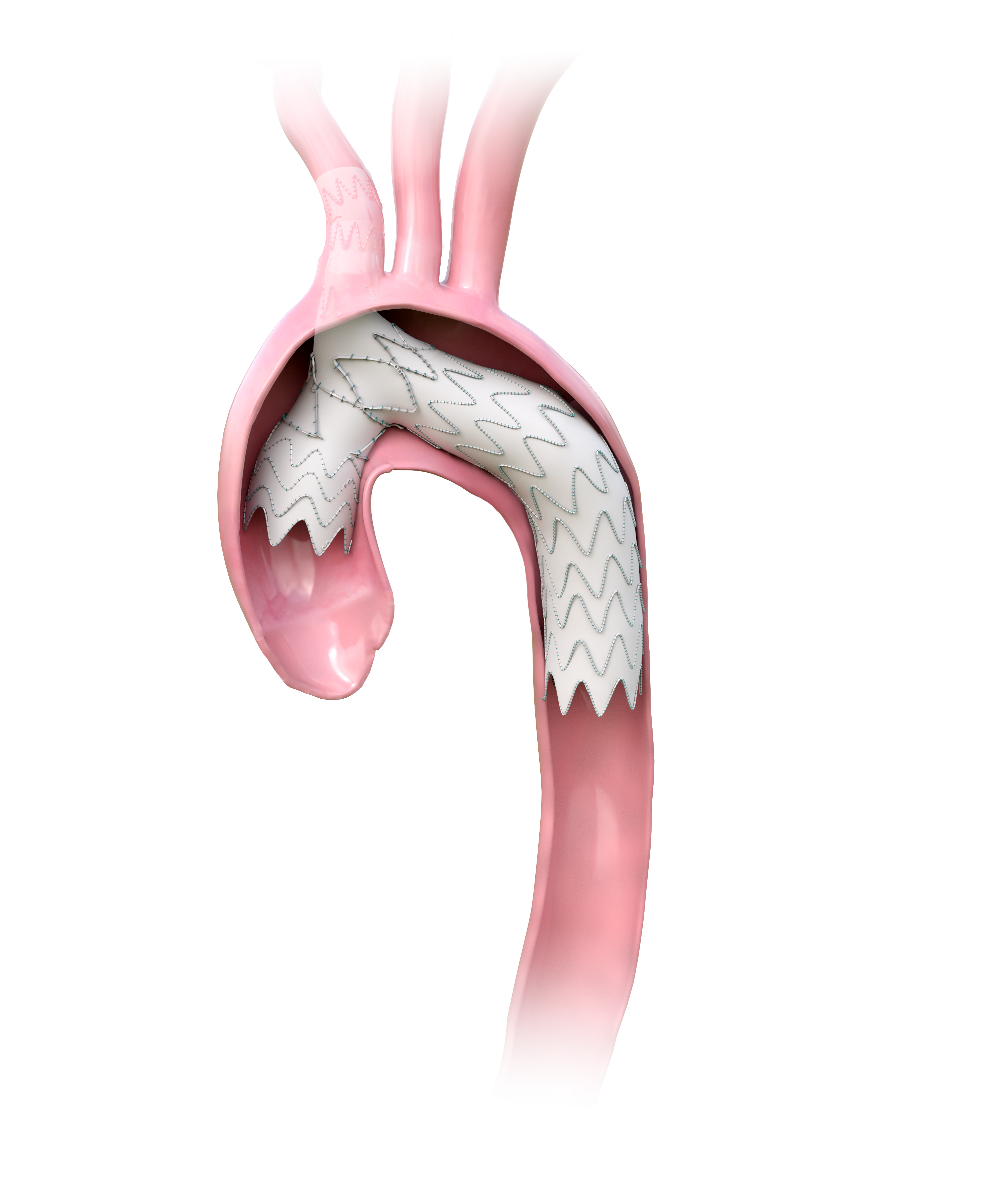
Advantages of the Artivion (Formerly CryoLife/Jotec) IBD Device Now from Artivion for Treating Common and Internal Iliac Artery Aneurysms; When Bell Bottom Technique; When IBD
Presented by: Lee Bouwman, MD, PhD, MSc
Role of Bare Metal AMDS Stents from Artivion (Formerly CryoLife/ Jotec) for Repair of Acute Type A Dissections: Indications and Results
Presented by: Wilson Y. Szeto, MD
Nexus OTS Endograft From Endospan for Treating Zone 0 and 1 Lesions of the Aortic Arch: How Does it Work and 3-Year Clinical Results: Status in the US
Presented by: Mario L. Lachat, MD/ Ross Milner, MD/ Andrew Holden, MBChB/ Daniel G. Clair, MD/ Nicola Mangialardi, MD
The Novel Artivion (Formerly CryoLife/ Jotec) Off-The Shelf (OTS) Inner Branched Device For TAAAs: Why is it Better Than Outer Branched OTS Devices: Are There Limitations
Presented by: Lee Bouwman, MD, PhD, MSc
Note: All products and indications are not available/approved in all markets.
Clinical Trials
A Prospective, Single Arm, Multi-Center Clinical Investigation to Evaluate the Safety and Effectiveness of AMDS* in the Treatment of Acute Debakey Type I Dissection Pivotal IDE Study. AMDS is intended for aortic repair, aortic remodeling, and re-expansion of the intimal flap within the ascending aorta, aortic arch, and into the descending aorta for patients with acute DeBakey I aortic dissection undergoing open surgical repair within 0 to 14 days after diagnosis.
Prospective, non-randomized, multi-center clinical investigation of the NEXUS Aortic Arch Stent Graft System (Nexus) for the treatment of thoracic aortic lesions involving the aortic arch with a proximal landing zone, native or previously implanted surgical graft, in the ascending aorta and with a brachiocephalic trunk native landing.
Note: CAUTION – Investigational device. Limited by Federal (or United States) law to investigational use. Except as otherwise noted, all trademarks are owned by Artivion, Inc. or its subsidiaries. AMDS is approved as an Investigational Device only and is not approved for commercial use in the US. The NEXUS® Aortic Arch System is manufactured by Endospan Ltd. and distributed by Artivion. NEXUS is a registered trademark of Endospan Ltd. NEXUS is approved as an Investigational Device only and is not approved for commercial use in the US. Not all products and indications are available or approved in all markets. © 2022 Artivion, Inc. All rights reserved.