On-X®
AORTIC HEART VALVE
A Valve for Life.
Product Highlights
- The only aortic mechanical valve with FDA, Health Canada, and CE Mark approval for Low INR of 1.5 – 2.0.1*
- Clinically proven to be safer with less anticoagulation resulting in >60% reduction in bleeding and no increase in thromboembolism.1-2*
- Unique material and design provide optimal hemodynamics compared with other mechanical and bioprosthetic aortic valves at ≥1 year. 1,3-7
*Reduce INR after 3 months of standard therapy.1
Product Overview
1. 90° Leaflet Opening

On-X Heart Valves
90° leaflet opening organizes flow1, reducing turbulence and thrombogenicity.3

All Other Bileaflet Valves
< 90º leaflet opening can lead to turbulent flow.3
2. Pure Pyrolytic Carbon
Designed to reduce thrombogenicity1-2

Pure Pyrolytic Carbon2
Silicon-Alloyed Pyrolytic Carbon2
3. Complete Annulus Support
Flared inlet organizes flow & prevents pannus1

Complete Annulus Support
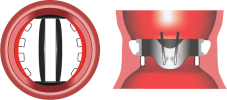
Incomplete Annulus Support


CYLINDRICAL END
For aortic valve sizes 19-25mm, the cylindrical end is used to find the correct fit.

REPLICA END
For aortic valve sizes 19-25mm, the replica end is used after sizing to ensure the fit of the valve without obstructing the coronary arteries.
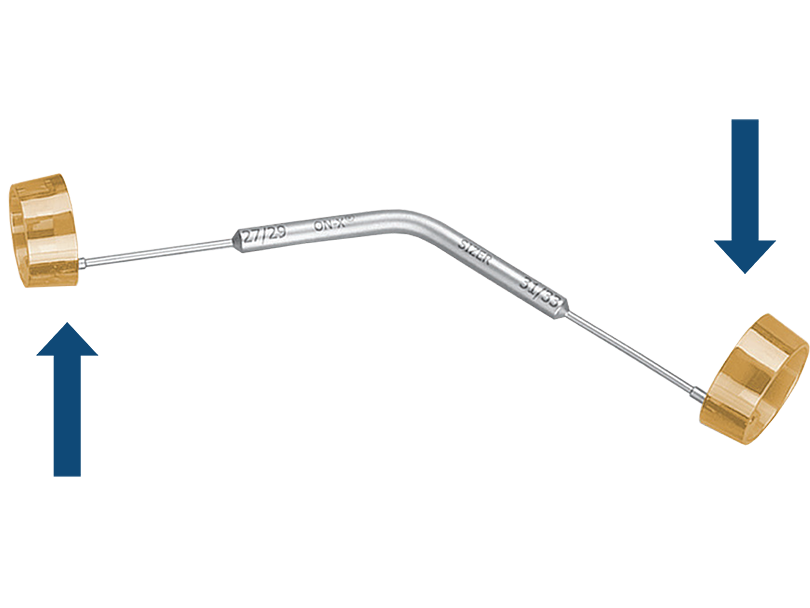
Conical End Sizer
For aortic valve sizes 27/29mm and 31/33mm, the conical end sizer is used to find the correct fit and assure intra-annular positioning.
The only artificial aortic valve to provide flow nearly the same as healthy human aortic valves is the On-X.
The bleeding risk associated with mechanical valves is almost nullified by a lower anticoagulation option [with the On-X Aortic Heart Valve], and the hemodynamics do not deteriorate over time as do bioprosthetic valves that place the patient in a watchful waiting phase toward the valve end of life.
Clinical Evidence
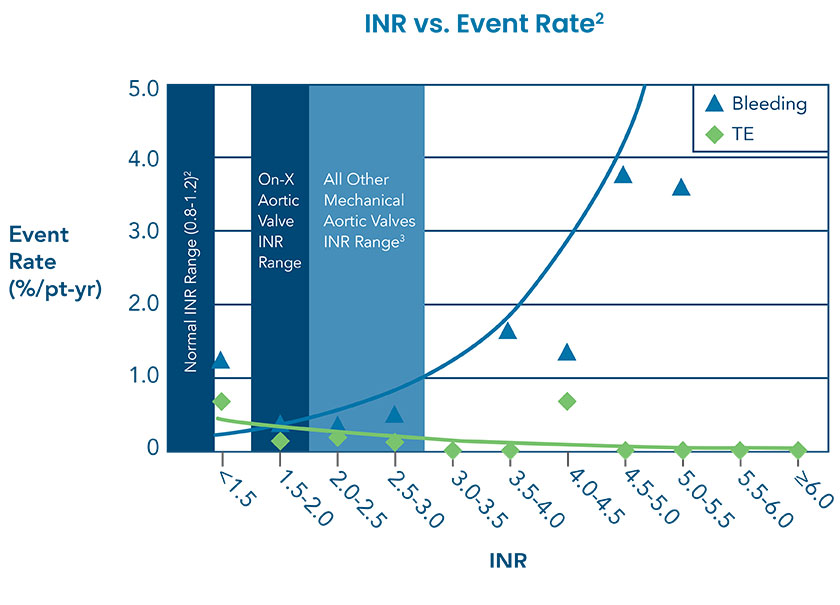
On-X Aortic Heart Valve: 50% Closer to a Normal INR†1
PROACT (Prospective Randomized On-X Anticoagulation Clinical Trial) determined that On-X Aortic Heart Valve patients with an INR of 1.5–2.0 had a >60% reduction in bleeding events and no increase in thromboembolism (TE) compared to patients with an INR of 2.0–3.0.*2 All other mechanical aortic valve anticoagulation should be managed at an INR of 2.0–3.0.3
† Normal INR is considered 1.0.1
*Reduce INR after 3 months post-operative standard therapy.4
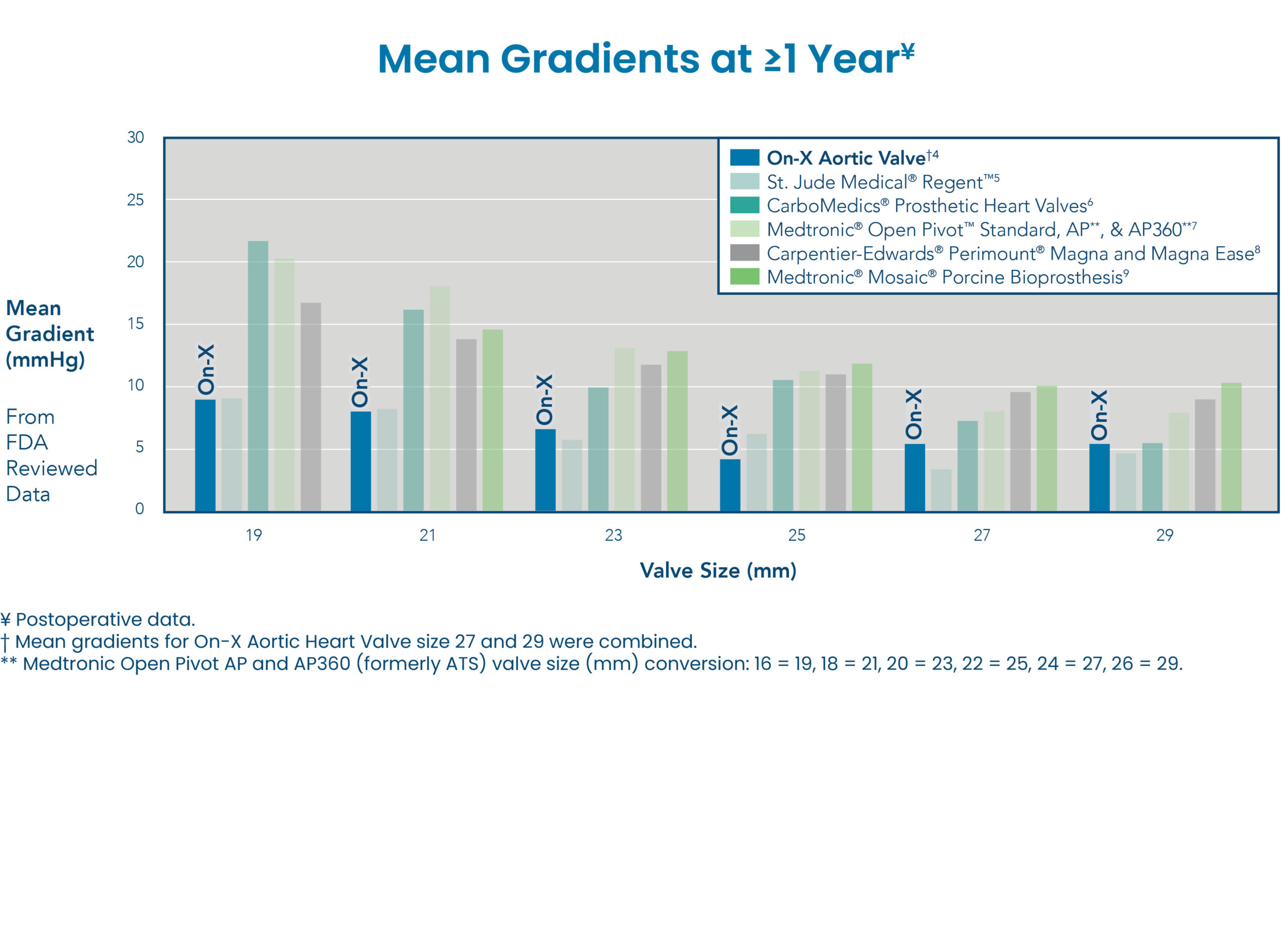
OPTIMAL HEMODYNAMICS
The On-X Aortic Valve provides optimal mean gradients compared with other bileaflet and bioprosthetic aortic valves at ≥1 year.4-9
Product Animation
The animation below demonstrates how the unique material and design features of the On-X Aortic Heart Valve promote laminar blood flow and reduce thrombogenicity.
Additional Resources
Explore additional resources for On-X below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- On-X Prosthetic Heart Valve Instructions for Use (01012277E).
- Puskas J, et. al. (2014). Reduced anticoagulation after mechanical aortic valve replacement: Interim results from the Prospective Randomized On-X® Valve Anticoagulation Clinical Trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg, 147(4), 1201-11.
- St. Jude Medical Physician’s Manual Mechanical Heart Valve.
- CarboMedics Prosthetic Heart Valve Instructions for Use.
- Medtronic Open Pivot Heart Valve Instructions for Use.
- Edwards Life Sciences Carpentier-Edwards PERIMOUNT Magna Ease Pericardial Aortic Bioprosthesis Model 3300TFX Instructions for Use.
- Medtronic Mosaic Porcine Bioprosthesis with Cinch Advanced Implant System Instructions for Use.
Product Overview
- On-X Prosthetic Heart Valve Instructions for Use (01012277E).
- LaGrange L, et. al. (1969, June 9-13). Compatibility of carbon and blood. Artificial Heart Program Conference, Washington, DC.
- Gerdisch M. et al. (2022) The role of mechanical valves in the aortic position in the era of bioprostheses and TAVR: Evidence-based appraisal and focus on the On-X valve. Prog Cardiovasc Dis. 72:31-40. doi: 10.1016/j.pcad.2022.06.001.
Clinical Evidence
- World Health Organization (1983). WHO Expert Committee on Biological Standardization. http://apps.who.int/iris/bitstream/10665/39217/1/WHO_TRS_687.pdf. Accessed on 06/29/2016.
- Puskas J, et. al. (2014). Reduced anticoagulation after mechanical aortic valve replacement: Interim results from the Prospective Randomized On-X® Valve Anticoagulation Clinical Trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg, 147(4), 1201-11.
- Nishimura RA, et. al. (2017) 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 135, e1159–95.
- On-X Prosthetic Heart Valve Instructions for Use (01012277E).
- St. Jude Medical Physician’s Manual Mechanical Heart Valve.
- CarboMedics Prosthetic Heart Valve Instructions for Use.
- Medtronic Open Pivot Heart Valve Instructions for Use.
- Edwards Life Sciences Carpentier-Edwards PERIMOUNT Magna Ease Pericardial Aortic Bioprosthesis Model 3300TFX Instructions for Use.
- Medtronic Mosaic Porcine Bioprosthesis with Cinch Advanced Implant System Instructions for Use.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1608.000. (2023-04)
| On-X Life Technologies, 1300 E Anderson Ln, Austin, TX 78752, US |

