On-X®
AORTIC HEART VALVE
A Valve for Life.
Product Highlights
- The only aortic mechanical valve with FDA, Health Canada, and CE Mark approval for Low INR of 1.5 – 2.0.1*
- Clinically proven to be safer with less anticoagulation resulting in 87% reduction in bleeding, with no increase in thromboembolism and no valve thrombosis.2*
- Unique material and design provide optimal hemodynamics compared with other mechanical and bioprosthetic aortic valves at ≥1 year. 1,3-7
*Reduce INR after 3 months of standard therapy.1
Product Overview
1. 90° Leaflet Opening

On-X Heart Valves
90° leaflet opening organizes flow1, reducing turbulence and thrombogenicity.3

All Other Bileaflet Valves
< 90º leaflet opening can lead to turbulent flow.3
2. Pure Pyrolytic Carbon
Designed to reduce thrombogenicity1-2

Pure Pyrolytic Carbon2
Silicon-Alloyed Pyrolytic Carbon2
3. Complete Annulus Support
Flared inlet organizes flow & prevents pannus1

Complete Annulus Support
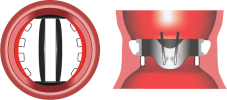
Incomplete Annulus Support


CYLINDRICAL END
For aortic valve sizes 19-25mm, the cylindrical end is used to find the correct fit.

REPLICA END
For aortic valve sizes 19-25mm, the replica end is used after sizing to ensure the fit of the valve without obstructing the coronary arteries.
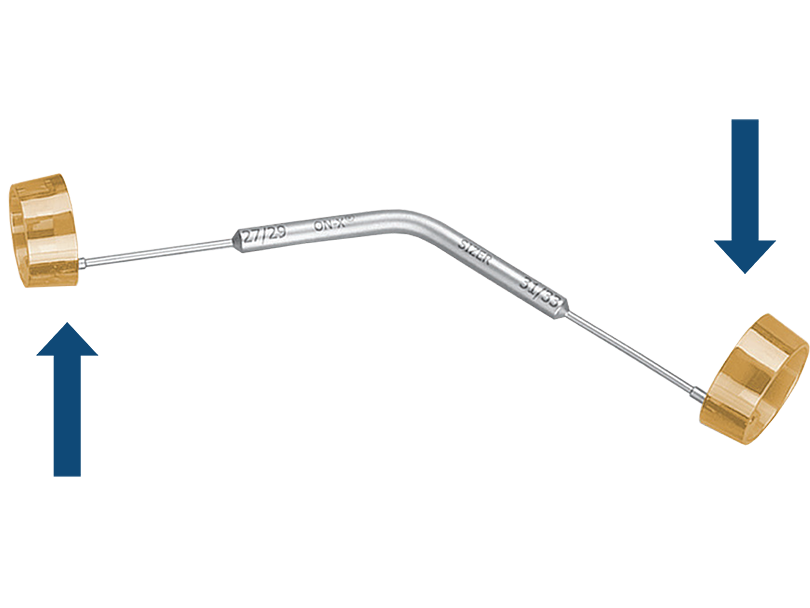
Conical End Sizer
For aortic valve sizes 27/29mm and 31/33mm, the conical end sizer is used to find the correct fit and assure intra-annular positioning.
The only artificial aortic valve to provide flow nearly the same as healthy human aortic valves is the On-X.
The bleeding risk associated with mechanical valves is almost nullified by a lower anticoagulation option [with the On-X Aortic Heart Valve], and the hemodynamics do not deteriorate over time as do bioprosthetic valves that place the patient in a watchful waiting phase toward the valve end of life.
Clinical Evidence
87% Lower Bleeding Risk with 50% Lower INR1
The On-X Aortic Heart Valve Post Approval Study (PAS) 5-year interim data determined that in a real-world setting, the On-X Aortic Valve managed at a low INR of 1.5-2.0 demonstrates an 87% reduction in major bleeding (MB) with no increase in thromboembolism (TE) and no valve thrombosis (VT) when compared to standard INR of 2.0-3.0.*1 All other mechanical aortic valve anticoagulation should be managed at an INR of 2.0-3.0.2
*Reduce INR after 3 months post-operative standard therapy.4
Open Access Publication – View here:
Low-dose warfarin with a novel mechanical aortic valve: Interim registry results at 5-year follow-up

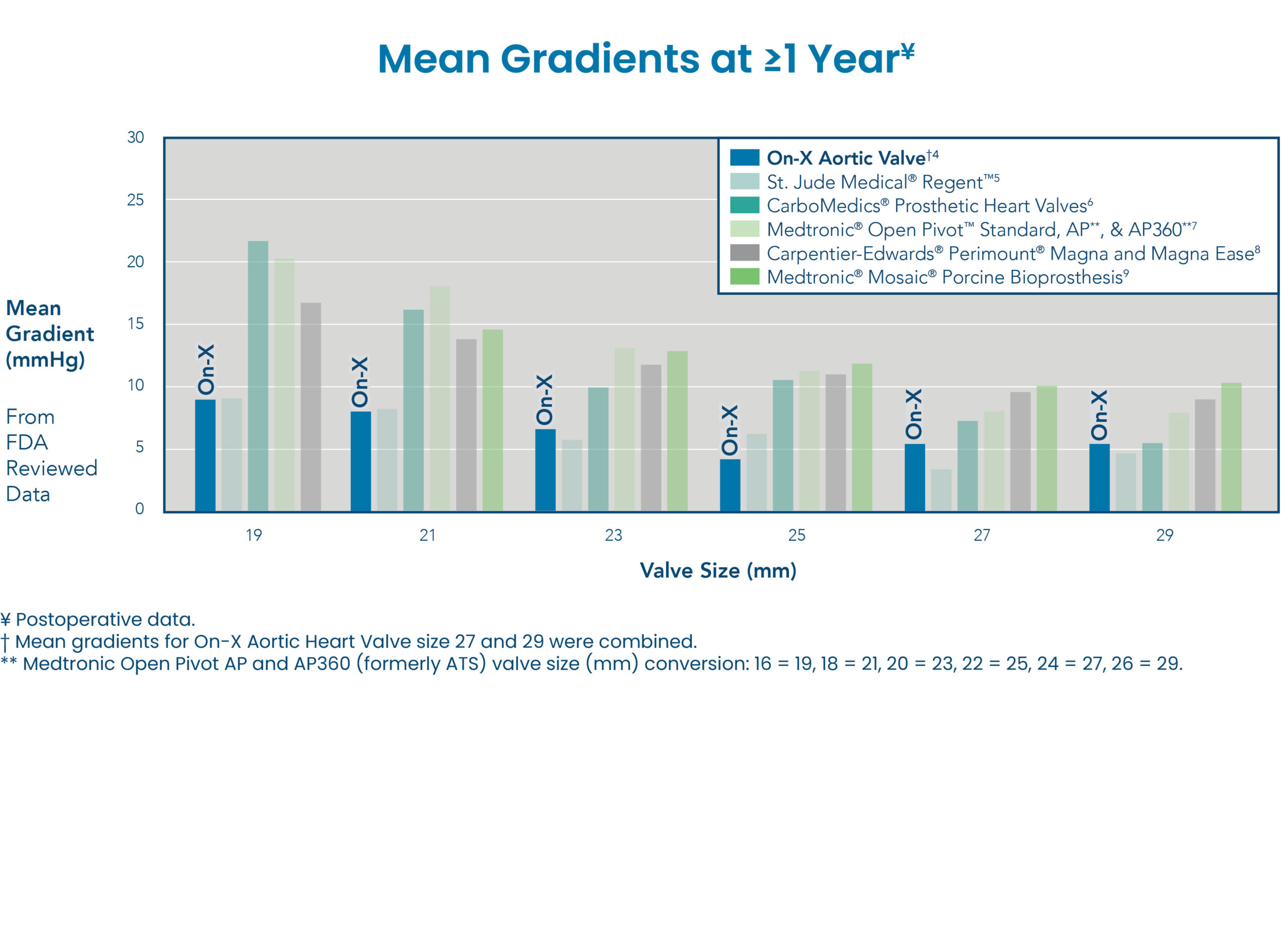
OPTIMAL HEMODYNAMICS
The On-X Aortic Valve provides optimal mean gradients compared with other bileaflet and bioprosthetic aortic valves at ≥1 year.4-9
Product Animation
Data-Driven Valve Selection for
SAVR Patients < 65
Explore additional data resources today!
Additional Resources
Explore additional resources for On-X below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- On-X Prosthetic Heart Valve Instructions for Use (01012277E).
- Gerdisch MW. et al. (2024) Low-Dose Warfarin with a Novel Mechanical Aortic Valve: Interim Registry Results at 5-Year Follow-Up. J Thorac Cardiovasc Surg. doi: https://doi.org/10.1016/j.jtcvs.2024.04.017.
- St. Jude Medical Physician’s Manual Mechanical Heart Valve.
- CarboMedics Prosthetic Heart Valve Instructions for Use.
- Medtronic Open Pivot Heart Valve Instructions for Use.
- Edwards Life Sciences Carpentier-Edwards PERIMOUNT Magna Ease Pericardial Aortic Bioprosthesis Model 3300TFX Instructions for Use.
- Medtronic Mosaic Porcine Bioprosthesis with Cinch Advanced Implant System Instructions for Use.
Product Overview
- On-X Prosthetic Heart Valve Instructions for Use (01012277E).
- LaGrange L, et. al. (1969, June 9-13). Compatibility of carbon and blood. Artificial Heart Program Conference, Washington, DC.
- Gerdisch M. et al. (2022) The role of mechanical valves in the aortic position in the era of bioprostheses and TAVR: Evidence-based appraisal and focus on the On-X valve. Prog Cardiovasc Dis. 72:31-40. doi: 10.1016/j.pcad.2022.06.001.
Clinical Evidence
- Gerdisch MW. et al. (2024) Low-Dose Warfarin with a Novel Mechanical Aortic Valve: Interim Registry Results at 5-Year Follow-Up. J Thorac Cardiovasc Surg. doi: https://doi.org/10.1016/j.jtcvs.2024.04.017.
- Nishimura RA, et. al. (2017) 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 135, e1159–95.
- Artivion data on file, weighted average of control groups from FDA Premarket Approval P000037 S030 and IDE trial G050208.
- On-X Prosthetic Heart Valve Instructions for Use (01012277E).
- St. Jude Medical Physician’s Manual Mechanical Heart Valve.
- CarboMedics Prosthetic Heart Valve Instructions for Use.
- Medtronic Open Pivot Heart Valve Instructions for Use.
- Edwards Life Sciences Carpentier-Edwards PERIMOUNT Magna Ease Pericardial Aortic Bioprosthesis Model 3300TFX Instructions for Use.
- Medtronic Mosaic Porcine Bioprosthesis with Cinch Advanced Implant System Instructions for Use.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MWENG0038.001 (2026-01)
| On-X Life Technologies, 1300 E Anderson Ln, Austin, TX 78752, US |

