
Neo EDE™
HYBRID ARCH DEVICE
CUSTOM-MADE DEVICE
Simplifying the Complex.
With 20 years of experience in leading innovation with the E-vita® Open platform in the Frozen Elephant Technique, we now offer you the possibility to redefine your FET procedures.
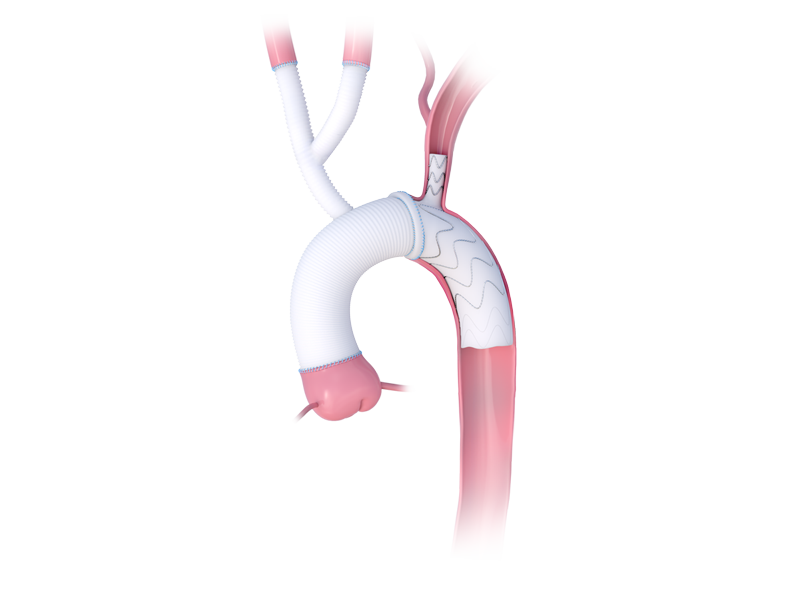
Product Highlights
A Customized Solution for Your Patient
- Outer or inner branches have been developed to facilitate this implantation into the supra-aortic vessels
- You can build a stented and vascular graft exactly to fit your patient’s anatomy
- Produced in 22 working days
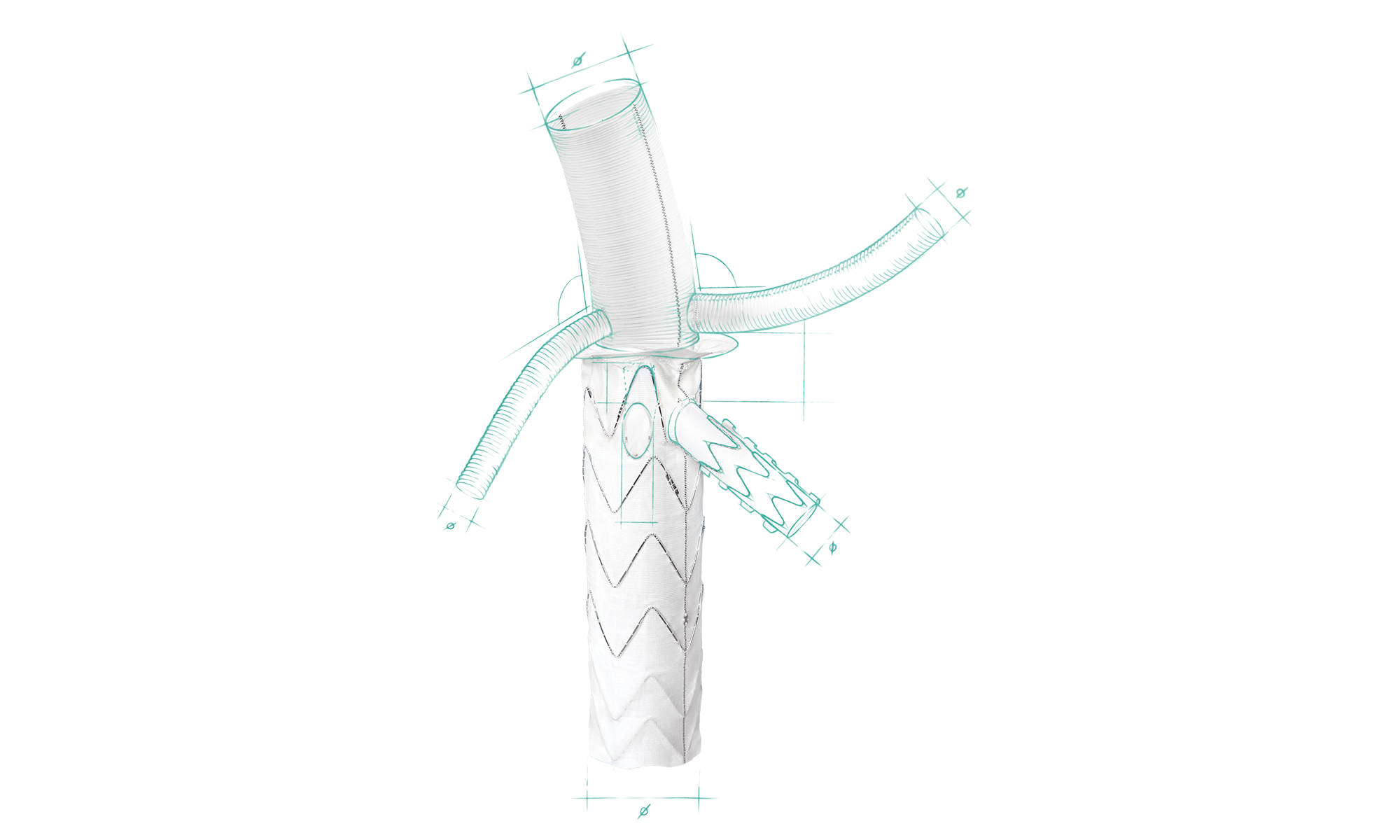
Product Overview
1. VASCULAR GRAFT PART
- Length: 5 mm – 100 mm
- Diameter: 24 mm – 40 mm
(20 mm – 40 mm for Inner branch and straight configurations)
2. VASCULAR BRANCHES*
• Diameter: 8, 10, 12, 14 mm
• Usable length: up to 100 mm
Customizable:
• Position
• Orientation
• Take-off-angles
*Max. of 4 branches in total

C01: Straight
C02: Branched

C03: Trifurcated
C04: Bifurcated
3. SUTURE COLLAR
• Width: 10 mm – 20 mm
• Angle: 0° – 45°

Straight

Conical

Angled
4. STENT GRAFT INNER BRANCH*
Fixed Parameters:
- Markers:
Distal end (1× Radiopaque marker)
Ostium (3× Ring markers)
Customizable:
- Take-off-angles
- Orientation
- Dimension (diameter and length)
- Position
*Max. of 2 inner branches on stent graft part


5. STENT GRAFT OUTER BRANCH*
Fixed Parameters:
- Markers: Distal end (1× Radiopaque marker) Ostium (3× Ring markers)
- Usable length: 40 mm
- Diameter: 11 or 15 mm will cover artery sizes 8.5 – 14 mm
Customizable:
- Position from Suture Collar: 10 mm +
- Orientation
*Max. of 1 outer branch on stent graft part

6. STENT GRAFT PART
- Length: 120 mm – 180 mm
- Diameter: 20 mm – 40 mm
(24 mm – 40 mm for Outer branch configurations) - Distal end: Straight cut (S) or Cutouts (C)

S: Straight cut

C: Cutouts
1. SHORTENED GRAFT PROTECTOR
To prevent tissue contact and improve visibility.
2. SINGLE-PULL MECHANISM
To deploy LSA stent & retract LSA tip consecutively.
3. ATRAUMATIC 18 FR LSA TIP
Ensures smooth insertion and retraction.

Product Animation
Caution: The availability of customized devices is subject to local regulatory guidelines. Neo EDE™ is a custom-made device for a particularly identified patient manufactured by JOTEC GmbH. All products and indications are not available / approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. JOTEC GmbH is a wholly owned subsidiary of Artivion, Inc. © 2025 Artivion, Inc. All rights reserved.
MWENG0062.000 (2025-06)
| JOTEC GmbH, Lotzenäcker 23, 72379 Hechingen, Germany |

