
E-xtra Design MultiBranch
STENT GRAFT SYSTEM
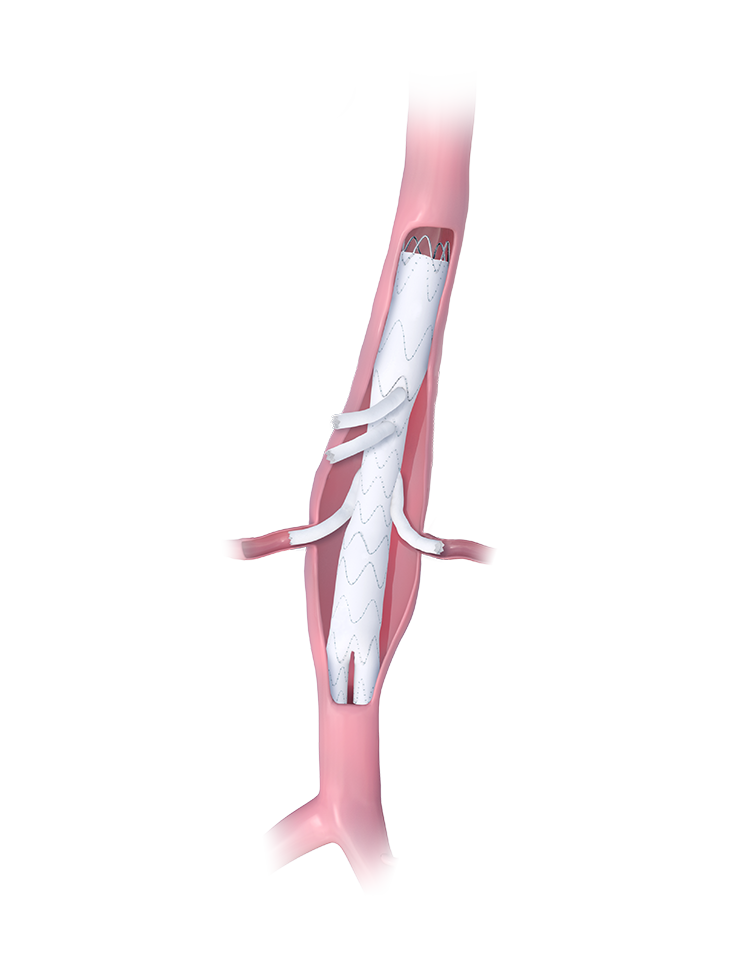
Custom-Made Device
Designed for Patient-Specific Anatomies
The E-xtra Design MultiBranch Stent Graft System is indicated for the endovascular treatment of patients with type I, II, III, IV or V thoracoabdominal aneurysms according to the Crawford classification or supra-, para- or juxtarenal abdominal aortic aneurysms or dissections extending to the thoracoabdominal aorta.
Product Highlights
- Produced in only 22 working days
- Can be built with different features, for example outer branches, inner branches, semi branches, twin branches, and scallops
- Diameters, lengths, widths, and orientations are patient-specific
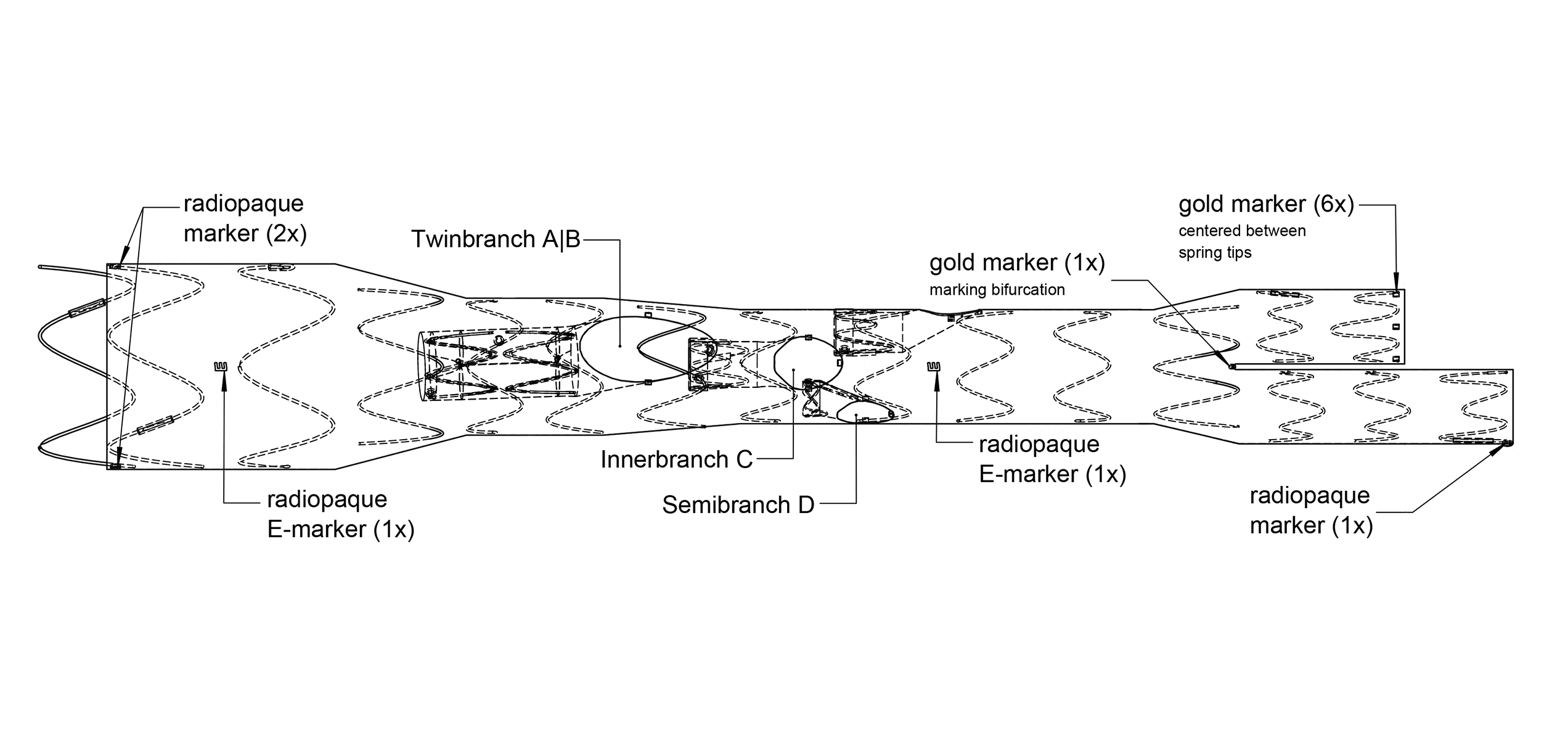
Product Overview
1. SCALLOP
Can be positioned proximally or distally
L and B: lengths and widths are patient-specific

2. OUTER BRANCH
Ø 6 mm, L=15 mm
Ø 7 mm, L=16 mm
Ø 8 mm, L=17 mm
Ante- or retrograde

3. TWIN BRANCH
Diameters, lengths and widths are patient-specific

4. INNER BRANCH
Ø 6 mm, L=20 mm
Ø 7 mm, L=20.5 mm
Ø 8 mm, L=21 mm
Ante- or retrograde

5. SEMI BRANCH
Diameters, lengths and widths are patient-specific
6. INTEGRATED BIFURCATION
(If needed)
Bifurcated shape: Ø10 or 13mm
1. FLEXIBLE CATHETER
(GRAFT COVER)
For accurate pushability and precise trackability.
2. RELEASE HANDLE
With a squeeze-to-release mechanism.
3. CONTROL HANDLE
4. RELEASE BUTTON WITH RETAINING RING

Clinical Evidence
The CONNECT General Study Information

CONNECT Study First Interim Results
- 4-6 Week Follow-Up

Inner branched complex aortic repair outcomes from a national multicenter registry using the E-xtra design platform (CELER Registry)
Journal: J Vasc Surg. 2023 Feb;77(2):338-346.
Authors: Simonte G, Isernia G, Gatta E, Neri E, Parlani G, Candeloro L, Schiavon S, Pagliariccio G, Cini M, Lenti M, Carbonari L, Ricci C.
Published: February 2023
EXTENT study: Medium-term outcomes of EXTra-design engineering inner-branch ENdografts for the treatment of complex aortic aneurysms from a multicenter collaboration
Journal: J Vasc Surg. 2024 Mar 11:S0741-5214(24)00431-2.
Authors: Abisi S, Zayed H, Frigatti P, Furlan F, Simonte G, Isernia G, Kuczmik W, Fattoum M, Halak M, Silverberg D, Gkoutzios P, Saha P; EXTENT Collaborators
Published: March 2024
The Semibranch: A New Tool for Complex Aortic Pathologies
Journal: J Endovasc Ther. 2023 Dec 22:15266028231219661
Authors: Oberhuber A, Simonte G, Isernia G, Schäfers J.
Published: December 2023
Additional Resources
Explore additional resources for Extra Design MultiBranch below. For further information or to contact a sales associate in your area, contact us.
Clinical Evidence
1. Fernandez, C. C. et al. Standard off-the-shelf versus custom-made multibranched thoracoabdominal aortic stent grafts. J Vasc Surg 63, 1208-1215, doi:10.1016/j. jvs.2015.11.035 (2016).
2. Hu, Z. et al. Multibranched Stent-Grafts for the Treatment of Thoracoabdominal Aortic Aneurysms: A Systematic Review and Meta-analysis. J Endovasc Ther 23, 626-633, doi:10.1177/1526602816647723 (2016).
3. Data on file at JOTEC GmbH.
Caution: All products and indications are not available/approved in all markets. The availability of custom-made devices is subject to local regulatory guidelines. E-xtra Design MultiBranch Stent Graft System is a custom-made device for a particularly identified patient manufactured by JOTEC GmbH. All trademarks are owned by Artivion, Inc., or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MWENG0031.001 (2025-06)
| JOTEC GmbH, Lotzenäcker 23, 72379 Hechingen, Germany |

