
E-liac™
STENT GRAFT SYSTEM
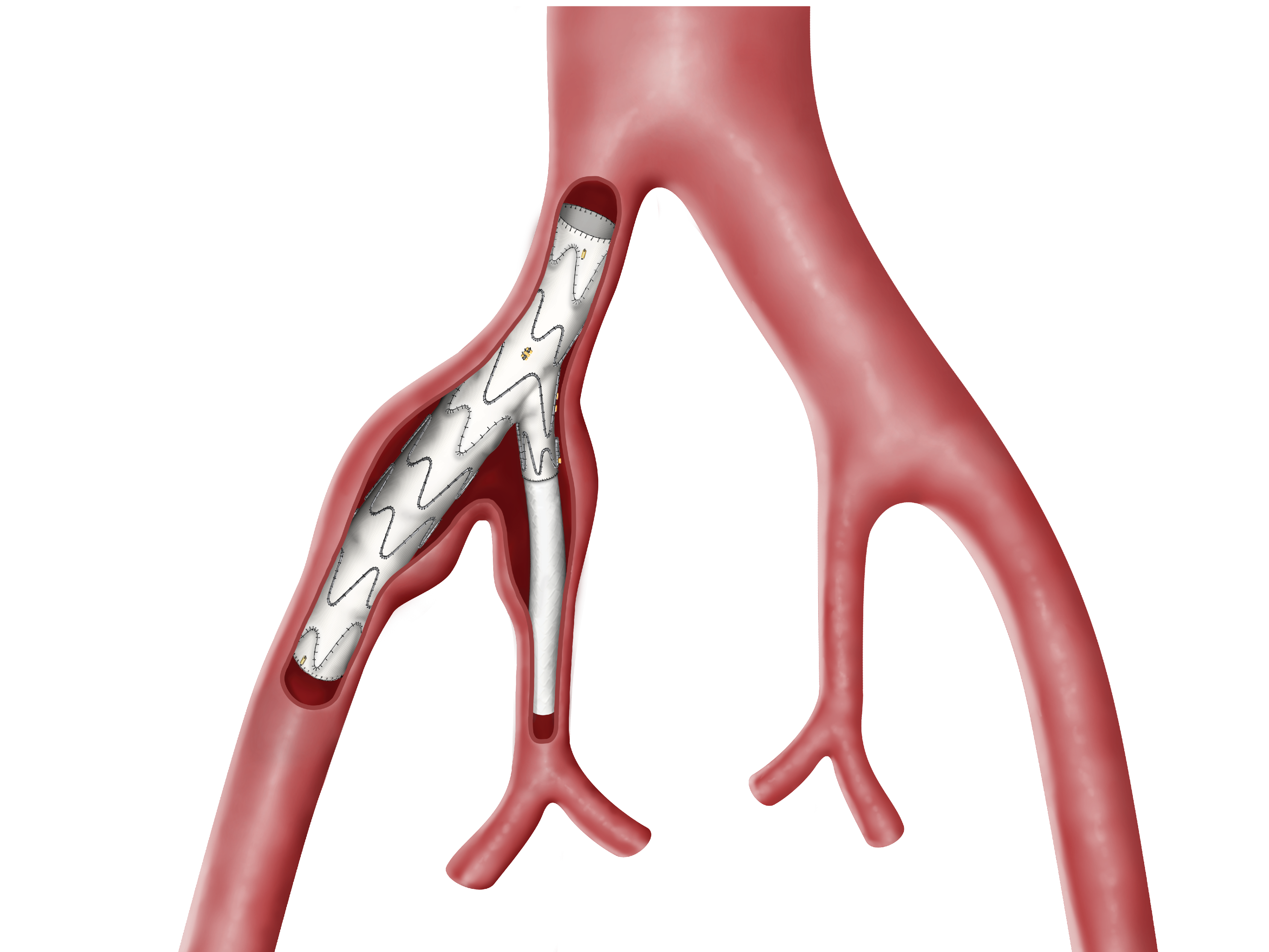
Hypogastric Artery Matters.
Product Highlights
- Indicated for both aorto-iliac and isolated iliac aneurysms.1
- Designed for a broad range of anatomies.
- High patency rates and low reintervention rates. 2-4
Product Overview
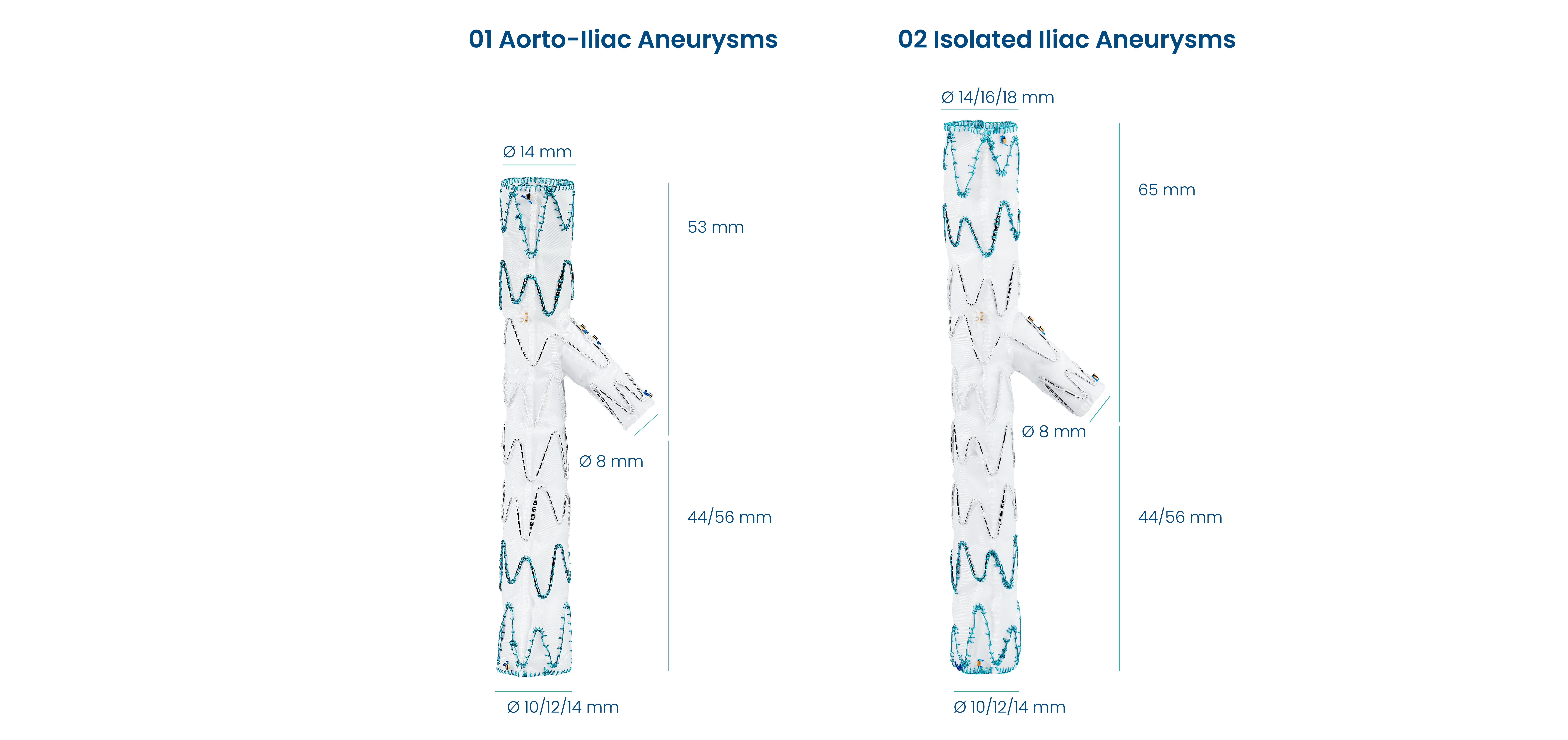
1. VISIBILITY
X-ray markers at various points allow safe positioning. The special E-marker indicates orientation of the side branch.

2. CONFORMABILITY
The asymmetric spring configuration allows good alignment to the vessel’s shape.

3. CONNECTION
A specially designed spring ensures the connection of the covered stent to the pre-cannulated side branch.

4. INDIVIDUALITY
Various proximal and distal diameters together with different lengths cover a wide range of anatomies for an individual treatment.
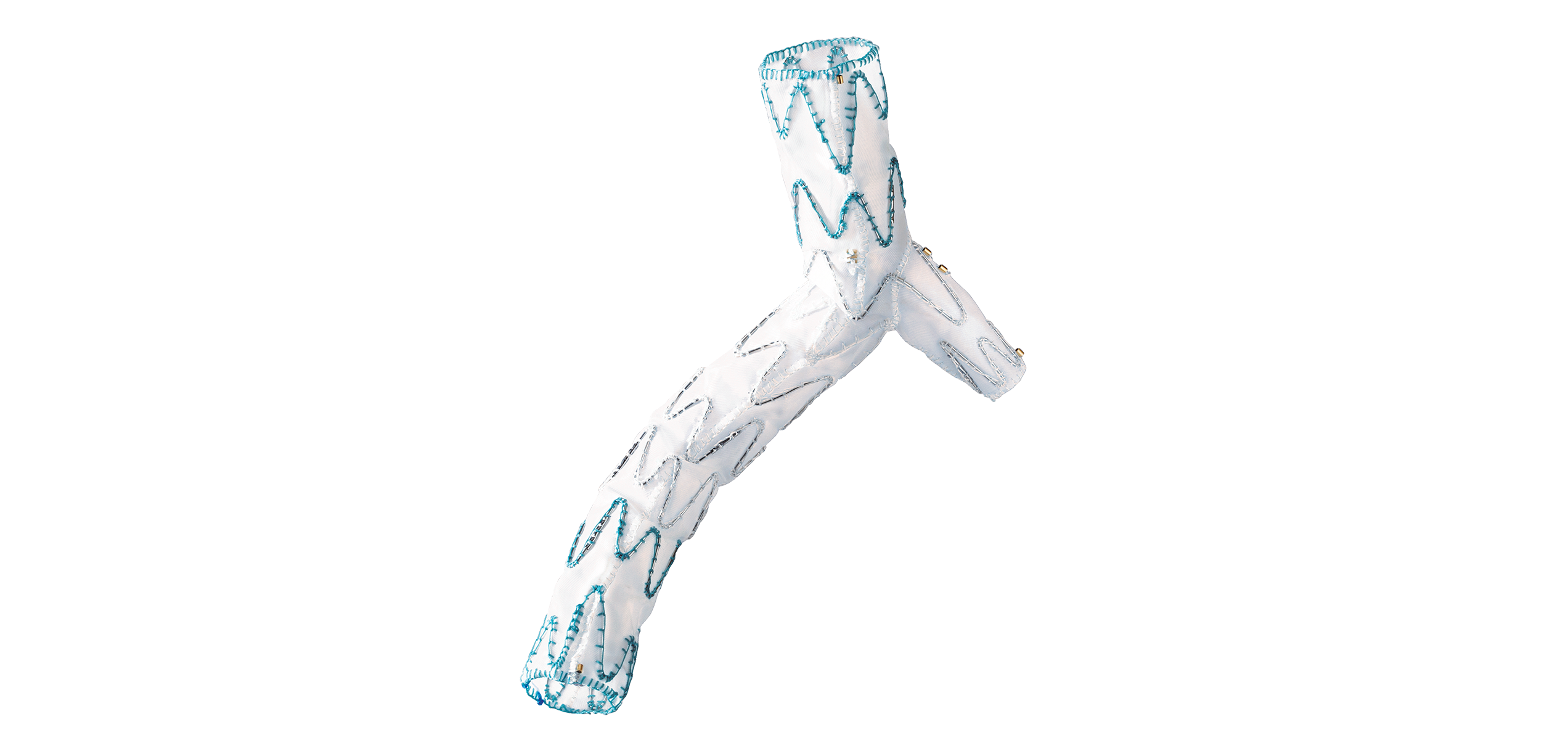
1. GUIDANCE
Axial and lateral lumen for guide wire introduction.

2. FLEXIBILITY
The catheter is designed for safe and precise advancement even in tortuous anatomies.
3. SMOOTH DELIVERY
The hydrophilic coating eases introduction and advancement of the system.
4. ORIENTATION
Tactile marking indicates orientation of the side branch and enables precise deployment of the stent graft.
5. EASY DEPLOYMENT
The Squeeze-to-Release mechanism allows gradual or continuous release with minimum effort.
6. CONTROL
The control handle secures the position of the delivery system during the procedure.
7. FUNCTIONALITY
The end cap of the delivery system contains various functions: guidance for the central and lateral guide wire as well as the release mechanism for the distal stent graft fixation.

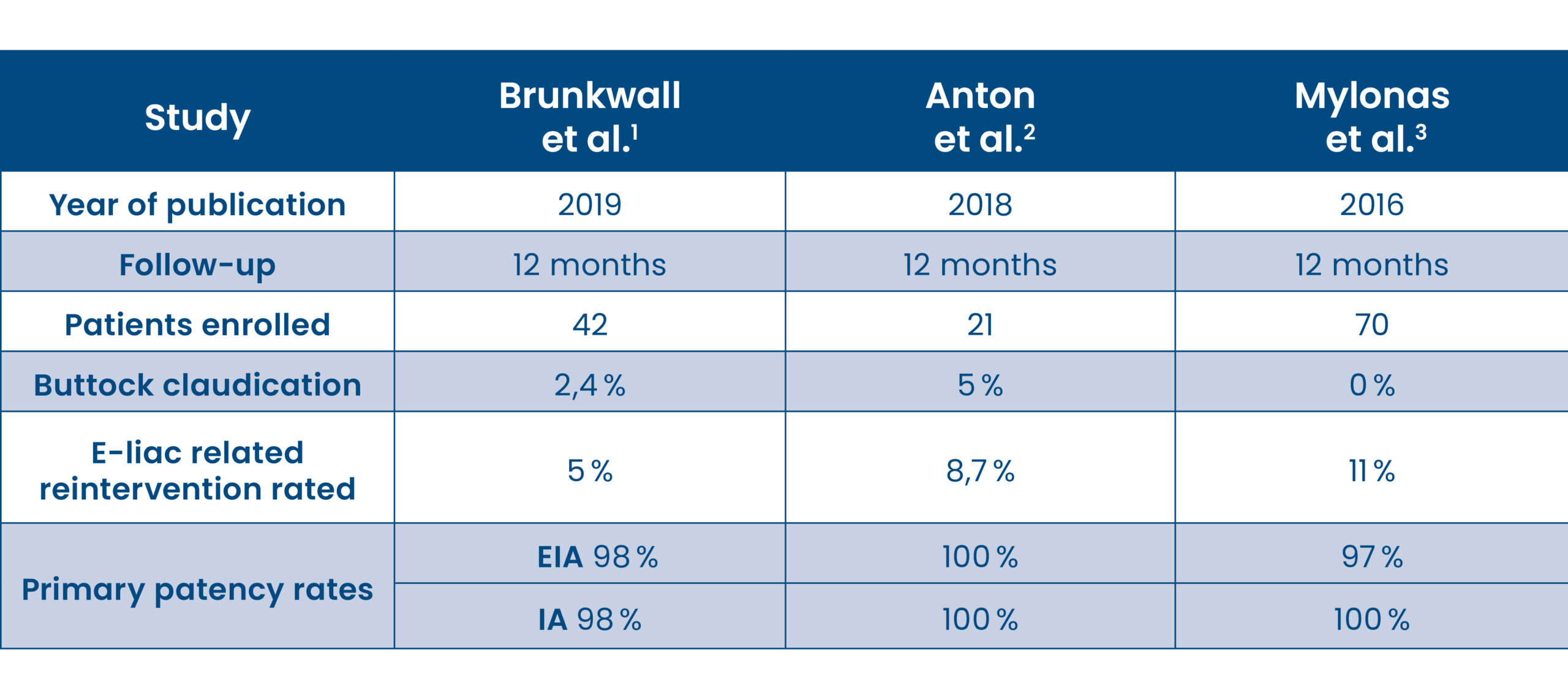
Clinical Data
The E-liac Stent Graft System has been tested in multiple studies where its safety and efficacy to maintain pelvic blood flow has been proven.
– Low reintervention rates
– High patency rates
Additional Resources
Explore additional resources for E-liac below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- E-liac Instructions for use (IFU 904225)
- Mylonas et.al. (2016). A multicenter 12-month experience with a new iliac side-branched device for revascularization of hypogastric arteries. J Vasc Surg, 64(6), 1652-1659.e1.
- Anton et.al. (2018). Initial Experience with the E-liac Iliac Branch Device for the Endovascular Aortic Repair of Aorto-iliac Aneurysm. Cardiovasc Intervent Radiol, 41(5), 683-6919.
- Brunkwall et.al. (2019). Prospective Study of the Iliac Branch Device E-liac in Patients with Common Iliac Artery Aneurysms: 12 Month Results. EJVS. doi:10.1016/j.ejvs.2019.06.020.
Clinical Evidence
- Brunkwall et.al. (2019). Prospective Study of the Iliac Branch Device E-liac in Patients with Common Iliac Artery Aneurysms: 12 Month Results. EJVS. doi:10.1016/j.ejvs.2019.06.020.
- Anton et.al. (2018). Initial Experience with the E-liac Iliac Branch Device for the Endovascular Aortic Repair of Aorto-iliac Aneurysm. Cardiovasc Intervent Radiol, 41(5), 683-6919.
- Mylonas et.al. (2016). A multicenter 12-month experience with a new iliac side-branched device for revascularization of hypogastric arteries. J Vasc Surg, 64(6), 1652-1659.e1.
Bench test data on file at Jotec GmbH. Data are not indicative of clinical performance.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MWENG0021.001. (2024-06)
| JOTEC GmbH, Lotzenäcker 23, 72379 Hechingen, Germany |

