CryoVein®
SAPHENOUS VEIN
The Natural Choice for
Vascular Reconstruction.
Product Highlights
- The ideal solution for bypass below the knee in patients at risk of infection.1
- Easy to suture with superior handling characteristics compared to PTFE grafts.2
- Natural suturability and natural pulsatile flow to treat patients with Critical Limb Ischemia.2-3
Product Overview
1. Superior Quality
Artivion significantly exceeds FDA and AATB donor rejection criteria standards to benefit patient care and outcomes.1,2 Strict criteria standards and Artivion’s proprietary tissue processing provide superior quality allografts.
2. Exceptional Durability
CryoVein Saphenous Vein demonstrates exceptional durability for peripheral bypass with 1-year primary patency up to 87%.3
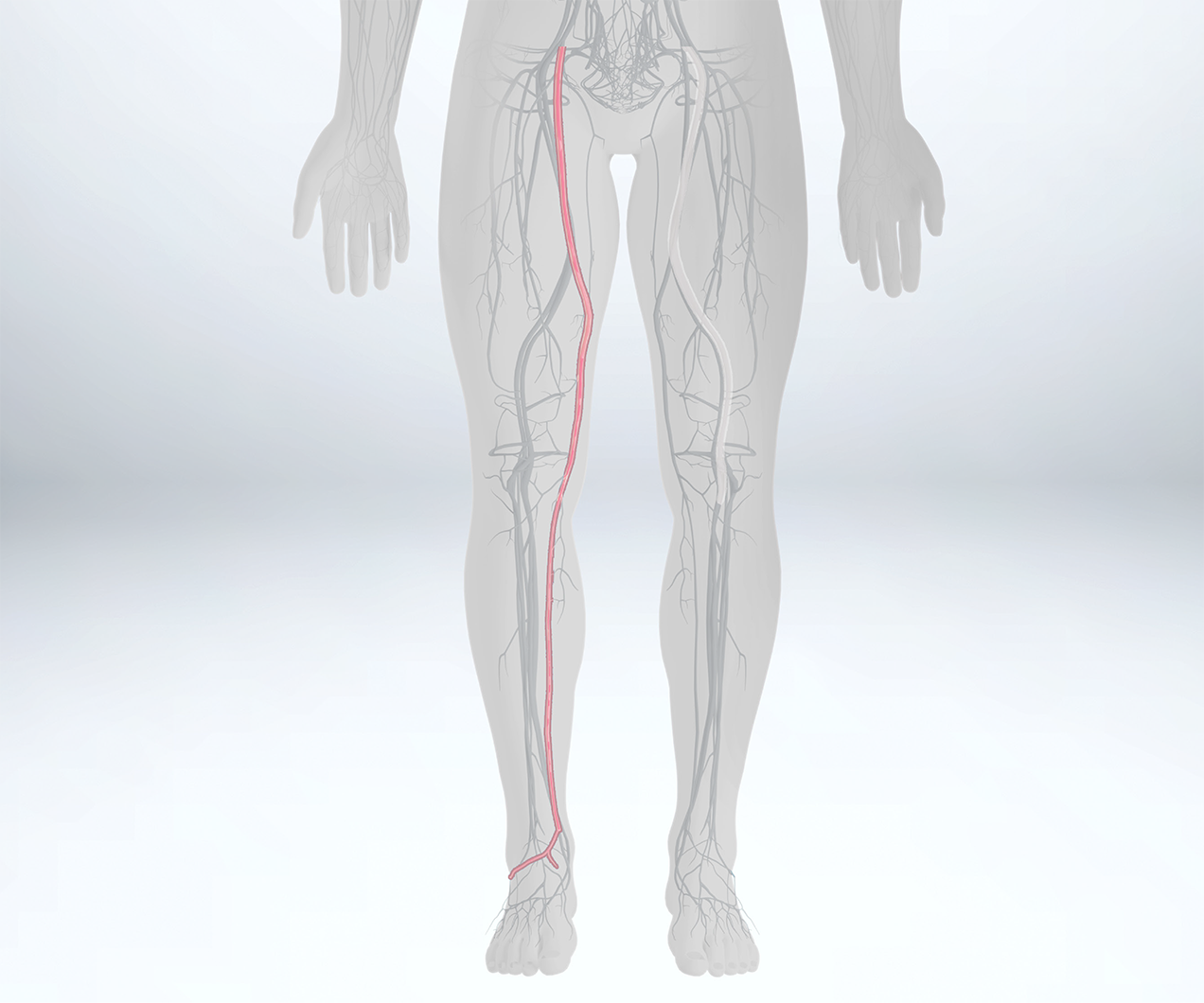
The majority of the patients we are treating are infrapopliteal because of all of the endovascular treatments. Usually, for above-the-knee patients, we provide endovascular treatments first and perform bypass only when these fail, so the majority of the patients we see are coming in for leg bypass. A lot of these patients are sick, and they have end-stage renal disease, diabetes, and hypertension. Often times do not have adequate autologous vein, or the vein was already used for a coronary artery bypass graft or other procedures. In these patients, I use CryoVein.
Clinical Evidence
The Natural Choice for Vascular Reconstruction
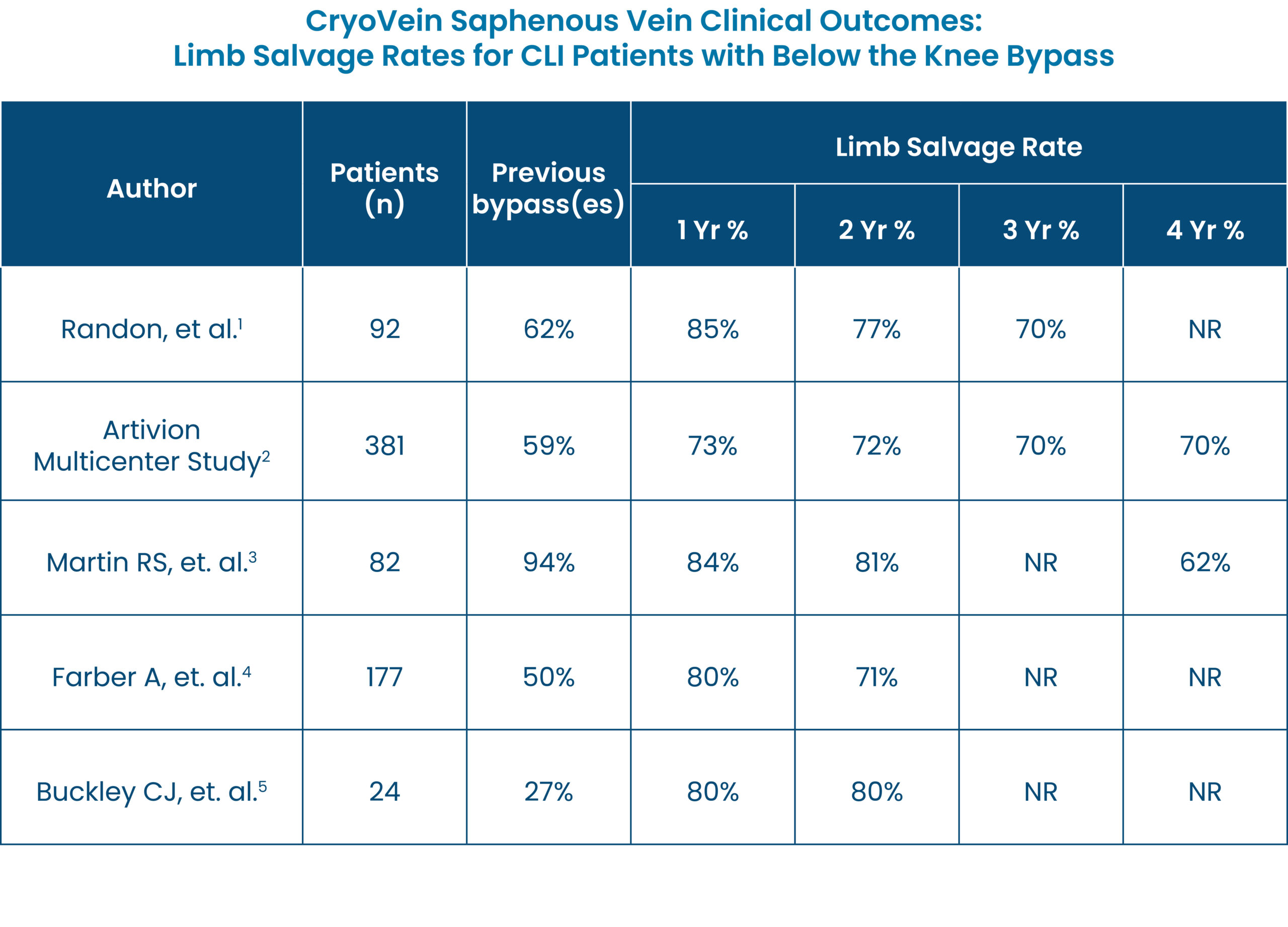
CryoVein Saphenous Vein is the ideal solution for below-knee revascularization in patients with Critical Limb Ischemia (CLI), patients with ulcers >2cm, or those without suitable autologous tissue for bypass.1 CryoVein Saphenous Vein demonstrates high limb salvage rates up to 70% at 4 years.1-5
Product Video
Additional Resources
Explore additional resources for CryoVein® Saphenous Vein below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Castier Y, et. al. (2005). Cryopreserved arterial allograft reconstruction for peripheral graft infection. J Vasc Surg, 41, 30-7.
- Martin RS, et. al. (1994). Cryopreserved Saphenous Vein Allografts for Below-Knee Lower Extremity Revascularization. Ann Vasc Surg, 219(6), 664-72.
- Bia D, et. al. (2007). Differential functional coupling between human saphenous cryoallografts and arteries: Importance of the arterial type and the biomechanical parameter evaluated. Artificial Organs, 31(11), 809–18.
Product Design Features
- The American Association of Tissue Banks Standards for Tissue Banking (Current Edition). https://www.aatb.org/standards. Accessed on February 9, 2023. Criteria reviewed at time of donation.
- FDA Donor Eligibility Regulations 21 CFR part 1271, Subpart C. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1271/subpart-C?toc=1. Accessed on February 9, 2023.
- Buckley CJ, et. al. (2000). Suggested treatment protocol for improving patency of femoral-infrapopliteal cryopreserved saphenous vein allografts. J Vasc Surg, 32(4), 731-8.
Clinical Evidence
- Randon C, et. al. (2010). Fifteen years of infrapopliteal arterial reconstructions with cryopreserved venous allografts for limb salvage. J Vasc Surg, 51(4), 869-77.
- Artivion Data on File (2001, March). CryoVein Saphenous Vein Allograft Infrainguinal Bypass Clinical Experience. ML0041.001.
- Martin RS, et. al. (1994). Cryopreserved Saphenous Vein Allografts for Below-Knee Lower Extremity Revascularization. Ann Vasc Surg, 219(6), 664-72.
- Farber A, et. al. (2003). Cryopreserved saphenous vein allografts in infrainguinal revascularization: analysis of 240 grafts. J Vasc Surg, 38(1), 15-21.
- Buckley CJ, et. al. (2000). Suggested treatment protocol for improving patency of femoral-infrapopliteal cryopreserved saphenous vein allografts. J Vasc Surg, 32(4), 731-8.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1603.000. (2023-04)
| Artivion, Inc., 1655 Roberts Blvd NW, Kennesaw, GA 30144, US |

