CryoVein® PC
PEDIATRIC CONDUIT
The Natural Choice for
Life-Restoring Cardiac
Connections.
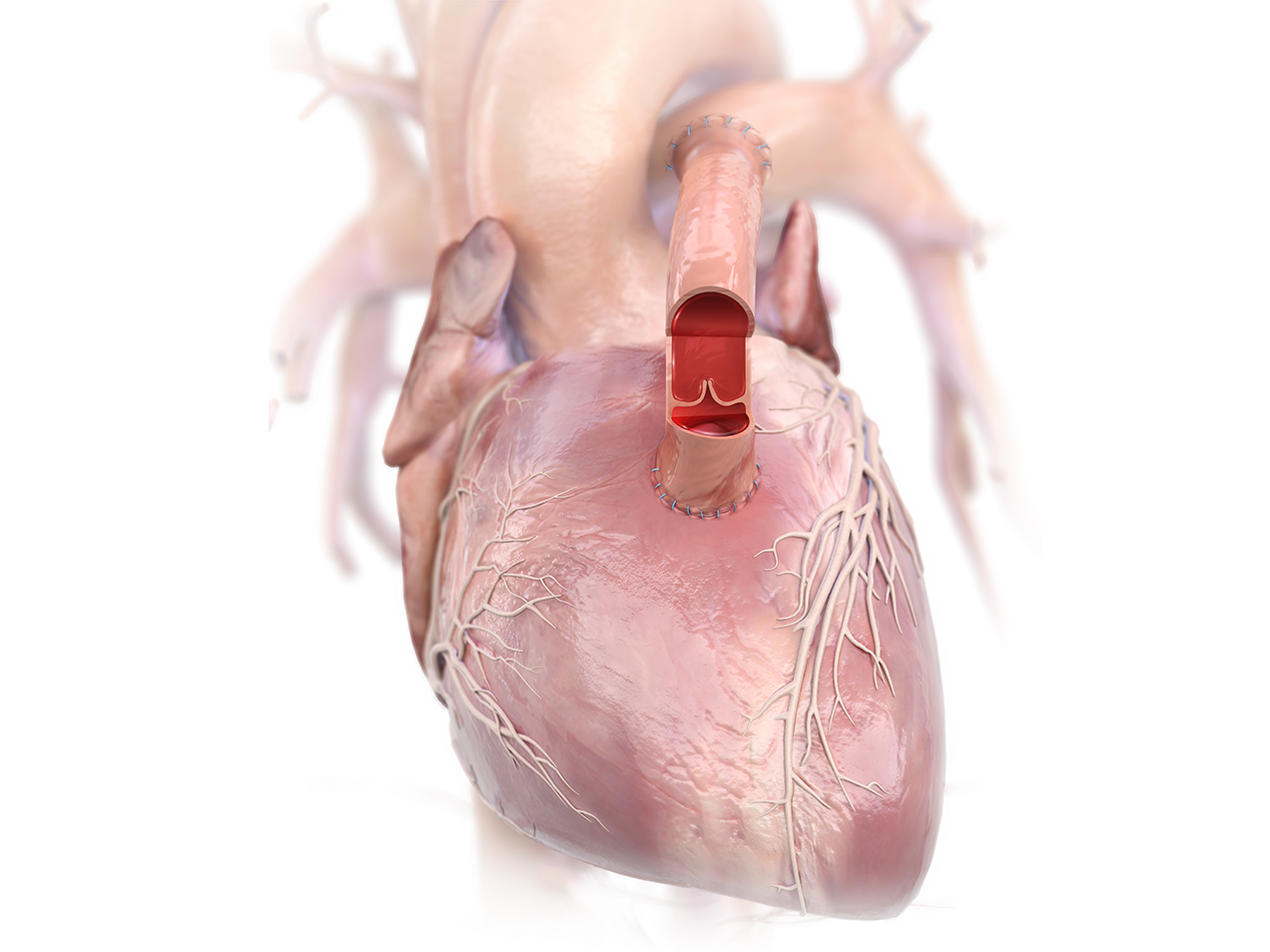
Product Highlights
- Improved pulmonary artery growth vs. PTFE graft.1
- Competent valve reduces regurgitation.2
- Infection resistant, easy to handle and suture, with minimal bleeding at suture line.2-3
Product Overview
1. Superior Quality
Artivion significantly exceeds FDA and AATB donor rejection criteria standards to benefit patient care and outcomes.1,2 Strict criteria standards and Artivion’s proprietary tissue processing provide superior quality allografts.
2. Natural Flexibility
CryoVein Pediatric Conduit demonstrates natural flexibility, allowing the allograft to stretch as the heart grows.3
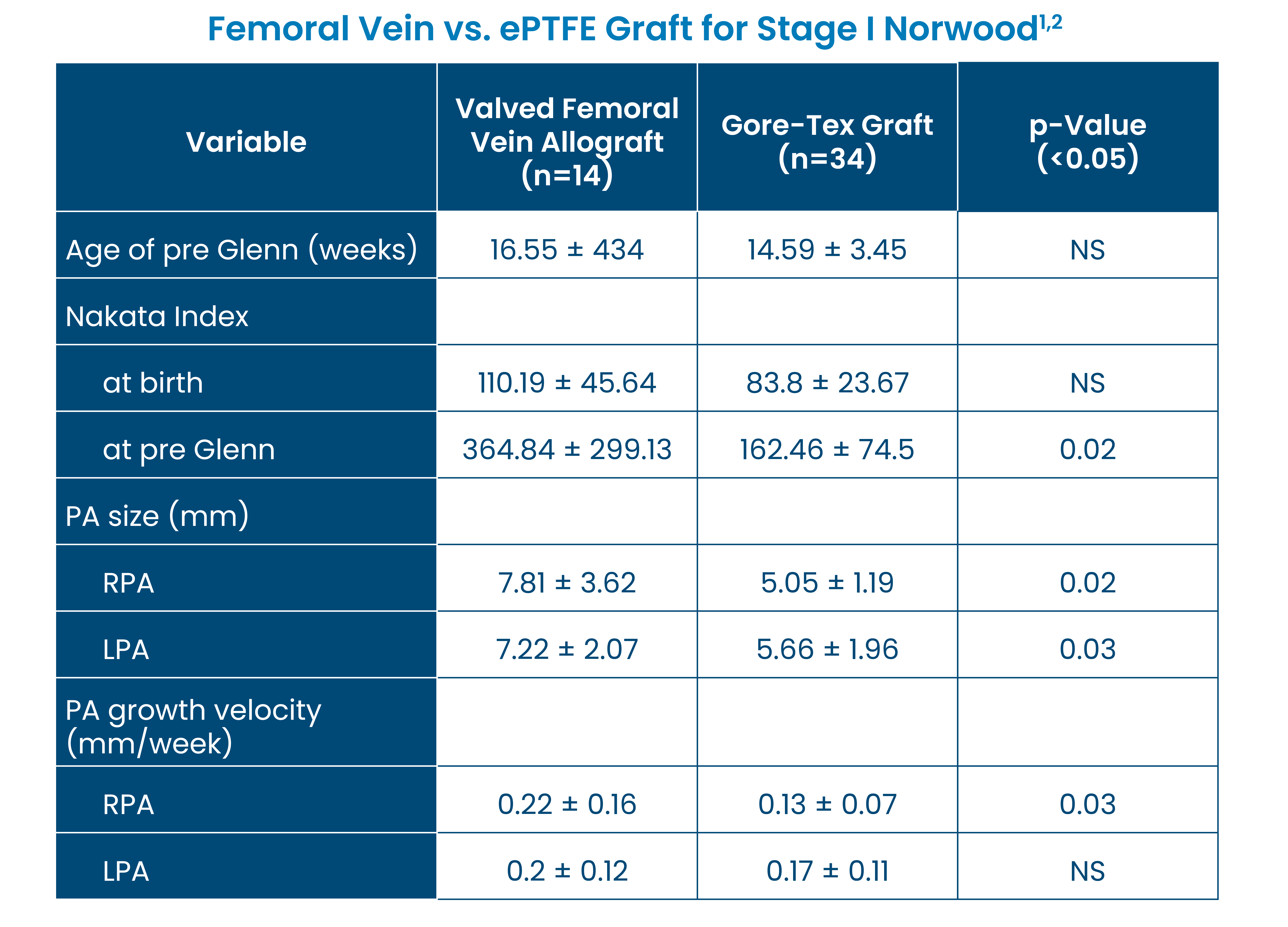
Clinical Evidence
Improved Pulmonary Artery Growth
Patients who received a femoral vein allograft compared to an ePTFE graft for a Stage I Norwood procedure demonstrated significantly better growth and development of branch pulmonary arteries and improved Nakata Index.1,2
Additional Resources
Explore additional resources for CryoVein® PC Pediatric Conduit below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Briceno-Medina M, et. al. (2018). Femoral vein homograft as Sano shunt results in improved pulmonary artery growth after Norwood operation. Cardiol Young, 28, 118-25.
- Bogáts GCA, et. al. (1996). Modified Blalock-Taussig shunt using allograft saphenous vein: Six Years experience. Annals Thor Surg, 61, 58-62.
- Takeuchi K, et. al. (2006). Evaluation of valved saphenous vein homograft as right ventricle-pulmonary artery conduit in modified stage I Norwood operation. Interact Cardio Vasc Thorac Surg, 5(4), 345-8.
Product Design Features
- The American Association of Tissue Banks Standards for Tissue Banking (Current Edition). https://www.aatb.org/standards. Accessed on February 9, 2023. Criteria reviewed at time of donation.
- FDA Donor Eligibility Regulations 21 CFR part 1271, Subpart C. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1271/subpart-C?toc=1. Accessed on February 9, 2023.
- Hamedani BA, et. al. (2012). Comparison between mechanical properties of human saphenous vein and umbilical vein. BioMedical Engineering OnLine, 11(1), 59-74.
Clinical Evidence
- Briceno-Medina M, et. al. (2018). Femoral vein homograft as Sano shunt results in improved pulmonary artery growth after Norwood operation. Cardiol Young, 28, 118-25.
- Kumar TKS, et. al. (2017). Femoral Vein Homograft as Right Ventricle to Pulmonary Artery Conduit in Stage 1 Norwood Operation. The Society of Thoracic Surgeons Conference.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1600.000. (2023-04)
| Artivion, Inc., 1655 Roberts Blvd NW, Kennesaw, GA 30144, US |

