
CryoVein®
FEMORAL VEIN
The Natural Choice
for Infected Fields.
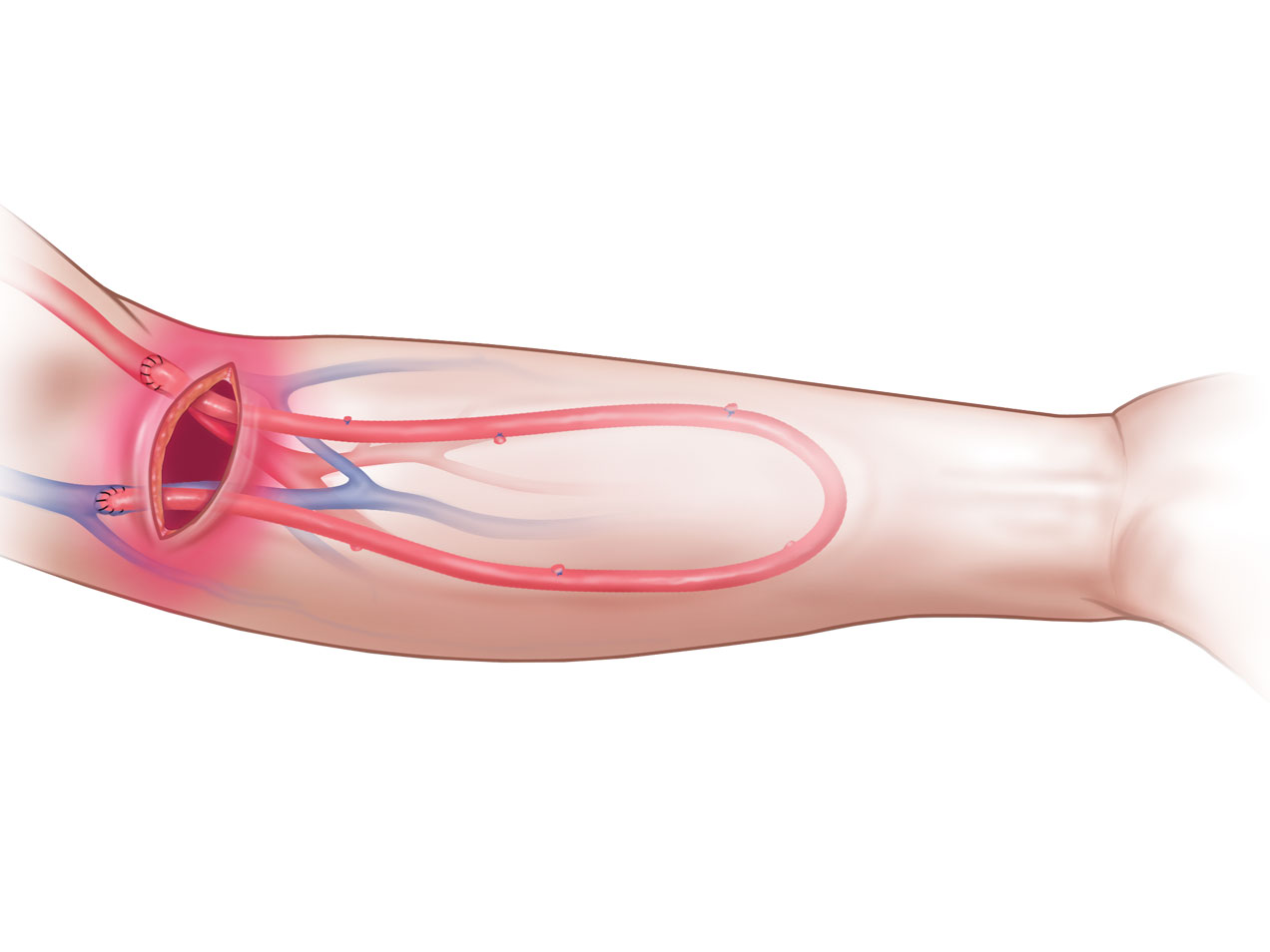
Product Highlights
- The ideal graft for patients with infected synthetic AV access grafts and patients at risk of infection.1-4
- Natural solution for patients with a limited number of AV access sites.1-2
- Outstanding durability and easy to suture.1,3,5
Product Overview
1. Superior Quality
Artivion significantly exceeds FDA and AATB donor rejection criteria standards to benefit patient care and outcomes.1,2 Strict criteria standards and Artivion’s proprietary tissue processing provide superior quality allografts.
2. Outstanding Durability
CryoVein Femoral Vein demonstrates outstanding durability for AV access with 2-year cumulative patency up to 72%.3

We had a very difficult patient with multiple failures with [AV] grafts in his arms, this gentleman was rather young and had very limited options for further access procedures. We put a cryopreserved femoral vein in his right arm, his wound closed and he’s been two to three years out now and has not had a recurrent infection. He’s delighted, I’m delighted, it was a very good option for him.
Clinical Evidence
Exceptional Resistance to Infection
- Up to 35% of synthetic arteriovenous (AV) grafts become infected, putting patients at risk of losing the limited number of viable AV access sites.1,2
- CryoVein Femoral Vein provides superior treatment to replace the infected graft, saving the viable access site, and demonstrates exceptionally low rates of reinfection, as shown across multiple studies.2-5
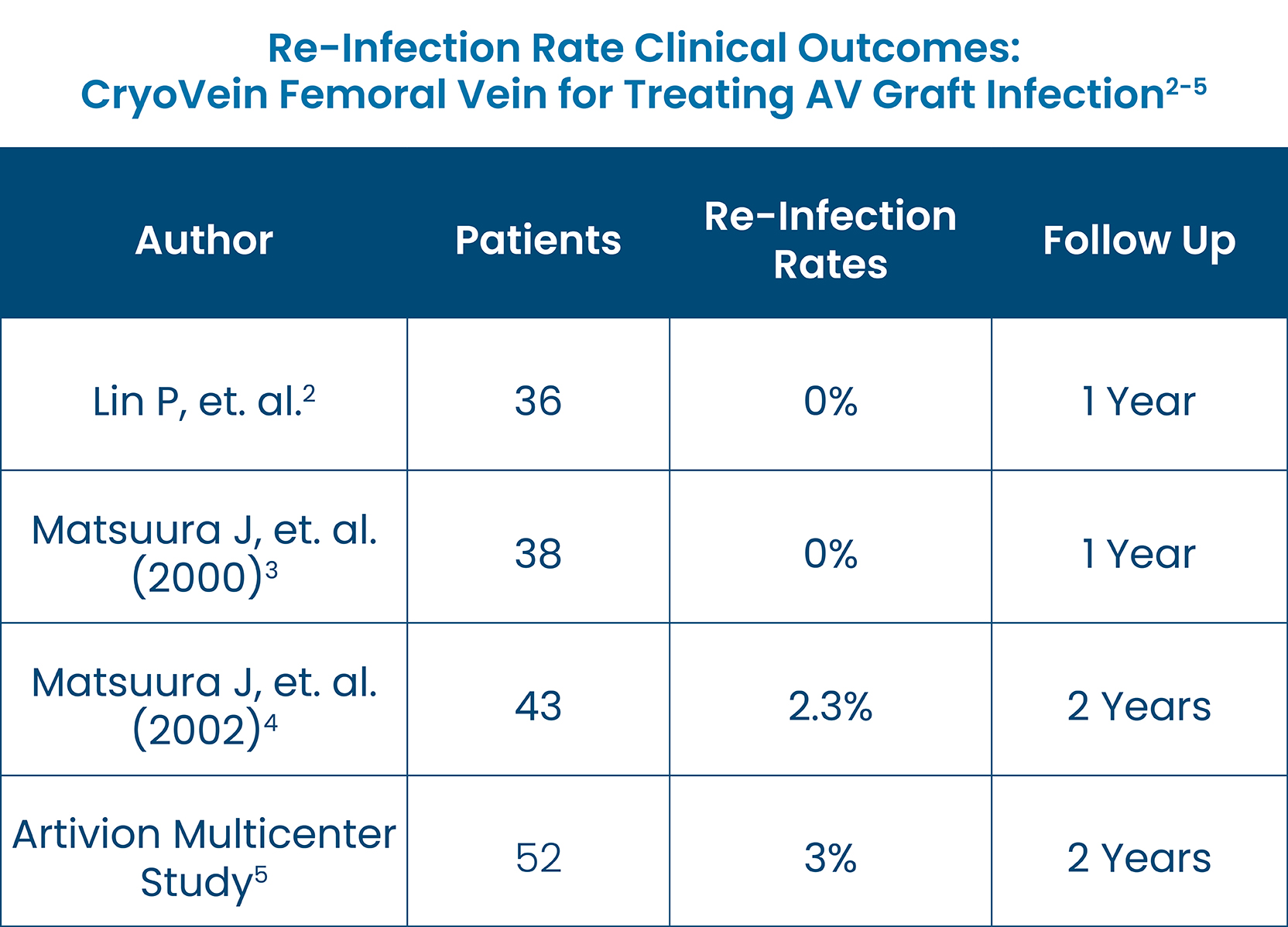
National Kidney Foundation Clinical Update: Infected Arteriovenous Graft (AVG) Access for the Hemodialysis Patient
Additional Resources
Explore additional resources for CryoVein® Femoral Vein below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Lin P, et. al. (2002). Management of infected hemodialysis access grafts using cryopreserved human vein allografts. Am J Surg, 184, 31-6.
- Matsuura J. (1999, November). Cryopreserved Human Femoral Vein: A New Option for Infected Access Grafts. Contemp Dial & Neph, 30-2.
- Brown KE, et. al. (2009). Arterial reconstruction with cryopreserved human allografts in the setting of infection: A single-center experience with midterm follow-up. J Vasc Surg, 49, 660-6.
- Matsuura J, et. al. (2000). Cryopreserved Femoral Vein Grafts for Difficult Hemodialysis Access. Ann Vasc Surg, 14, 50-5.
- Matsuura J, et. al (2002). Hemodialysis graft infections treated with cryopreserved femoral vein. Cardiovasc Surg, 10(6), 561-65.
Product Design Features
- The American Association of Tissue Banks Standards for Tissue Banking (Current Edition). https://www.aatb.org/standards. Accessed on February 9, 2023. Criteria reviewed at time of donation.
- FDA Donor Eligibility Regulations 21 CFR part 1271, Subpart C. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1271/subpart-C?toc=1. Accessed on February 9, 2023.
- Artivion Data on File (2001, September). CryoVein Femoral Vein Allograft Hemodialysis Access Clinical Experience. ML0101.
Clinical Evidence
- Akoh JA. (2009). Prosthetic arteriovenous grafts for hemodialysis. J Vasc Access, 10(3), 137-47.
- Lin P, et. al. (2002). Management of infected hemodialysis access grafts using cryopreserved human vein allografts. Am J Surg, 184, 31-6.
- Matsuura J. (1999, November). Cryopreserved Human Femoral Vein: A New Option for Infected Access Grafts. Contemp Dial & Neph, 30-2.
- Matsuura J, et. al. (2000). Cryopreserved Femoral Vein Grafts for Difficult Hemodialysis Access. Ann Vasc Surg, 14, 50-5.
- Matsuura J, et. al (2002). Hemodialysis graft infections treated with cryopreserved femoral vein. Cardiovasc Surg, 10(6), 561-65.
- Artivion Data on File (2001, September). CryoVein Femoral Vein Allograft Hemodialysis Access Clinical Experience. ML0101.000.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1615.000. (2023-04)
| Artivion, Inc., 1655 Roberts Blvd NW, Kennesaw, GA 30144, US |

