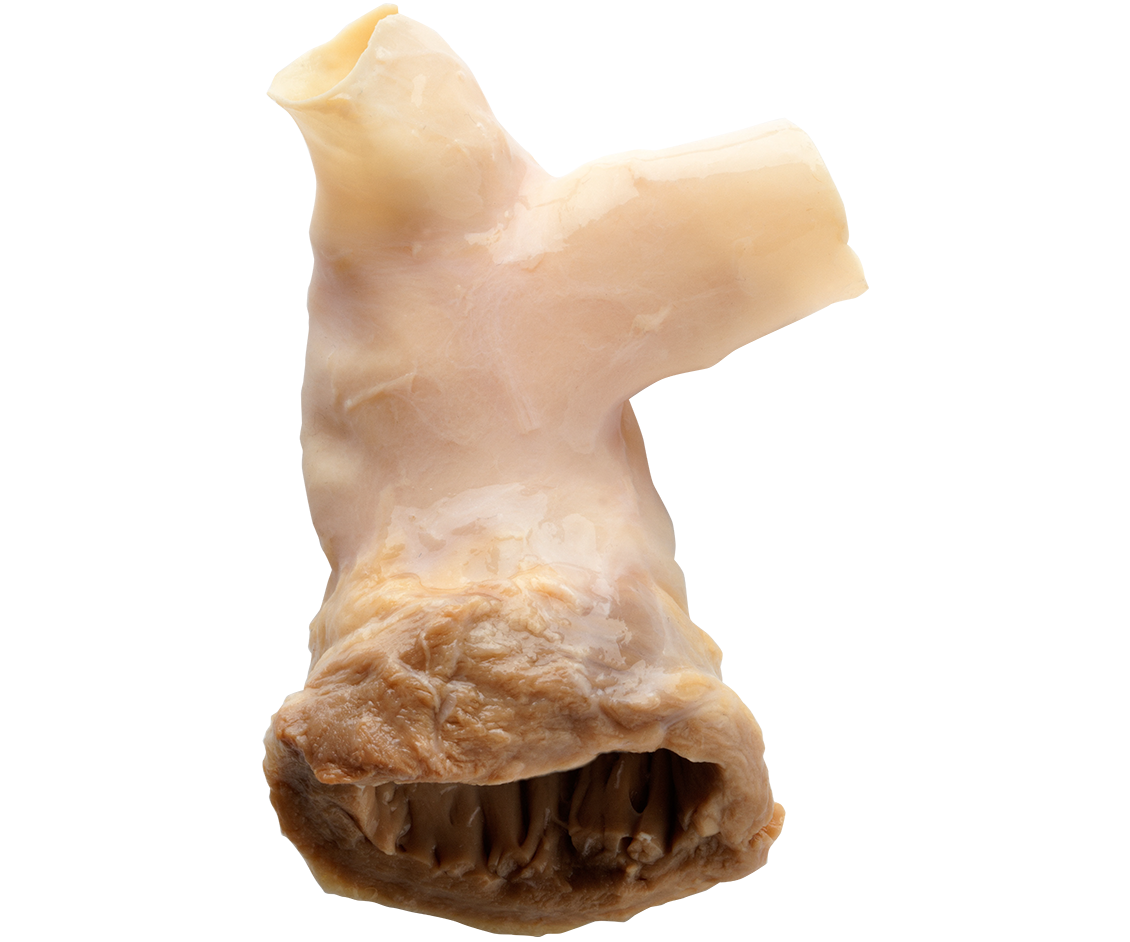
CryoValve® SG
PULMONARY HUMAN HEART VALVE
The Right Valve for
the Right Side.
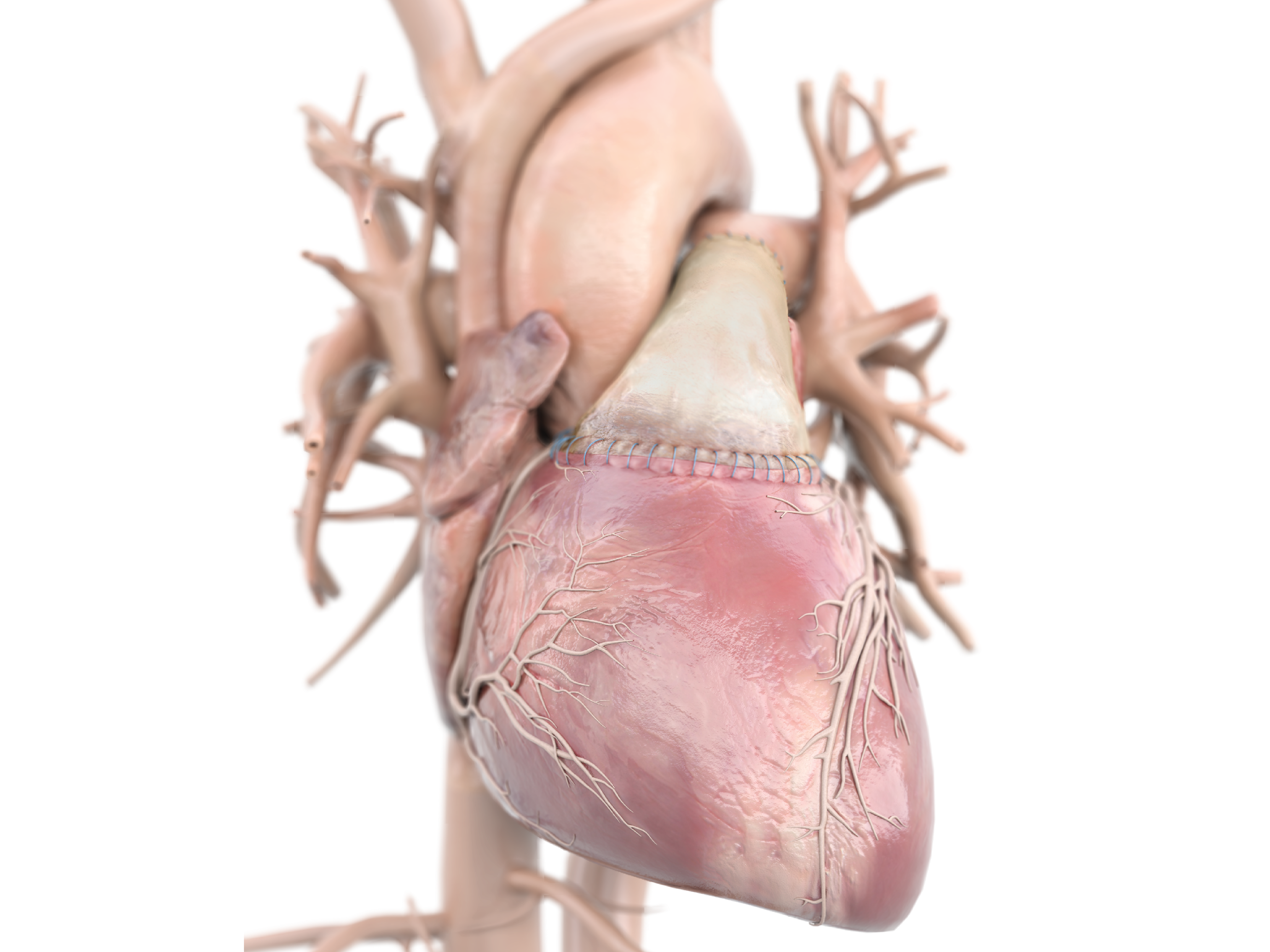
Product Highlights
- CryoValve SynerGraft(SG) Pulmonary Human Heart Valve is the first and only decellularized human heart valve to receive 510(k) clearance from the U.S. Food and Drug Administration.
- The SynerGraft process virtually eliminates the presence of allogeneic donor cells while maintaining the structural integrity of the biological matrix.1,2
- Excellent durability, optimal hemodynamics, and increased freedom from pulmonary insufficiency.3-5
Product Overview
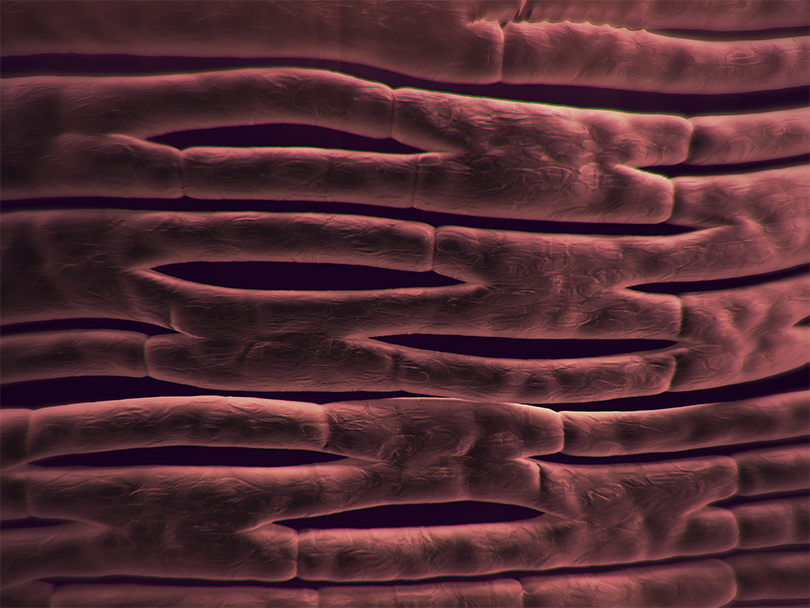
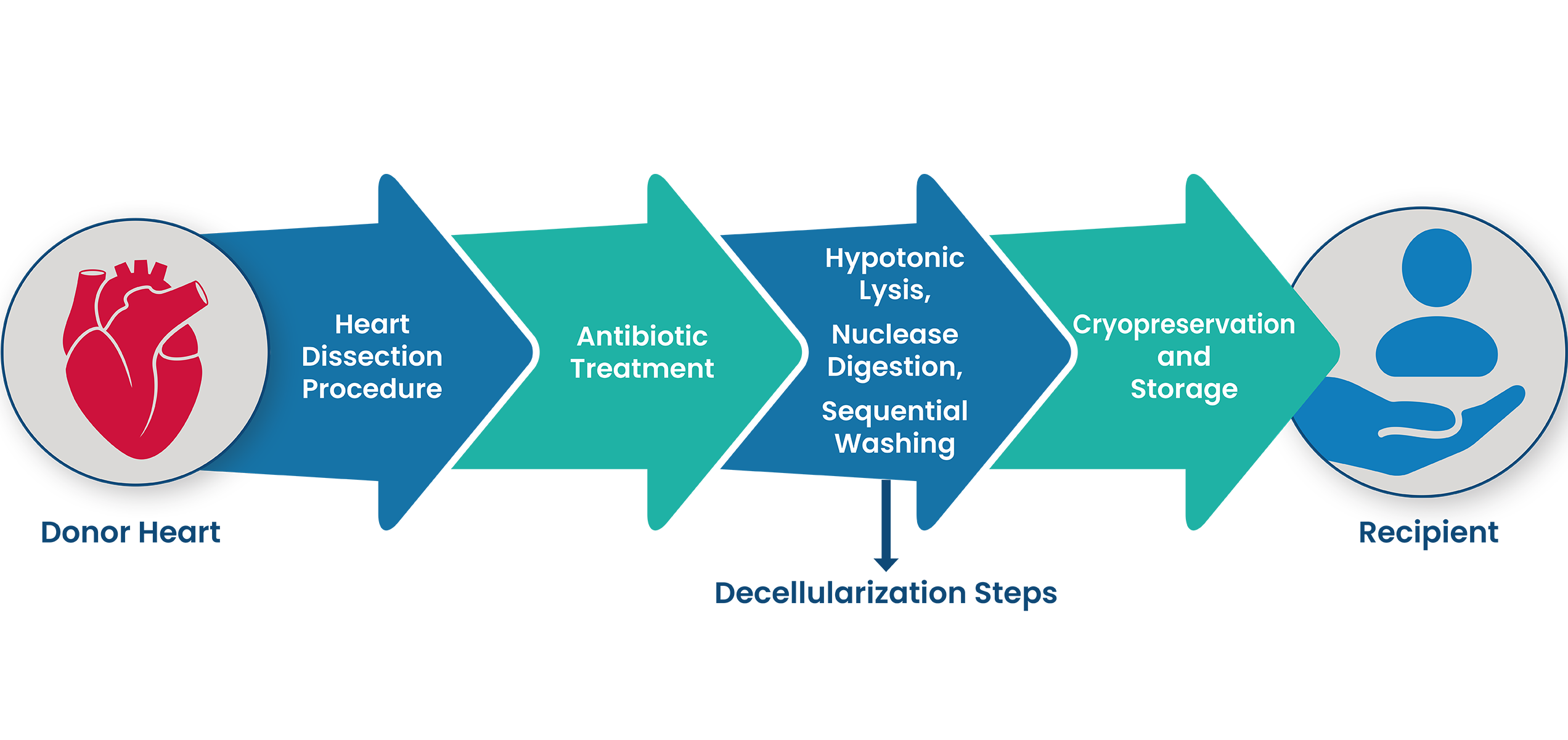
1. SYNERGRAFT DECELLULARIZATION PROCESS
The SynerGraft process includes hypotonic cell lysis, enzymatic digestion of nucleic acids, and sequential washing in a neutral buffer. These three steps work together to ensure maximal degradation and removal of donor cell material and nucleic acids without damaging structural proteins.1-3
There is evidence to support the use of decellularized homografts in terms of better longevity and less valve dysfunction. And therefore, my preference is to use SynerGraft decellularized pulmonary homografts [for the Ross procedure].
Clinical Evidence
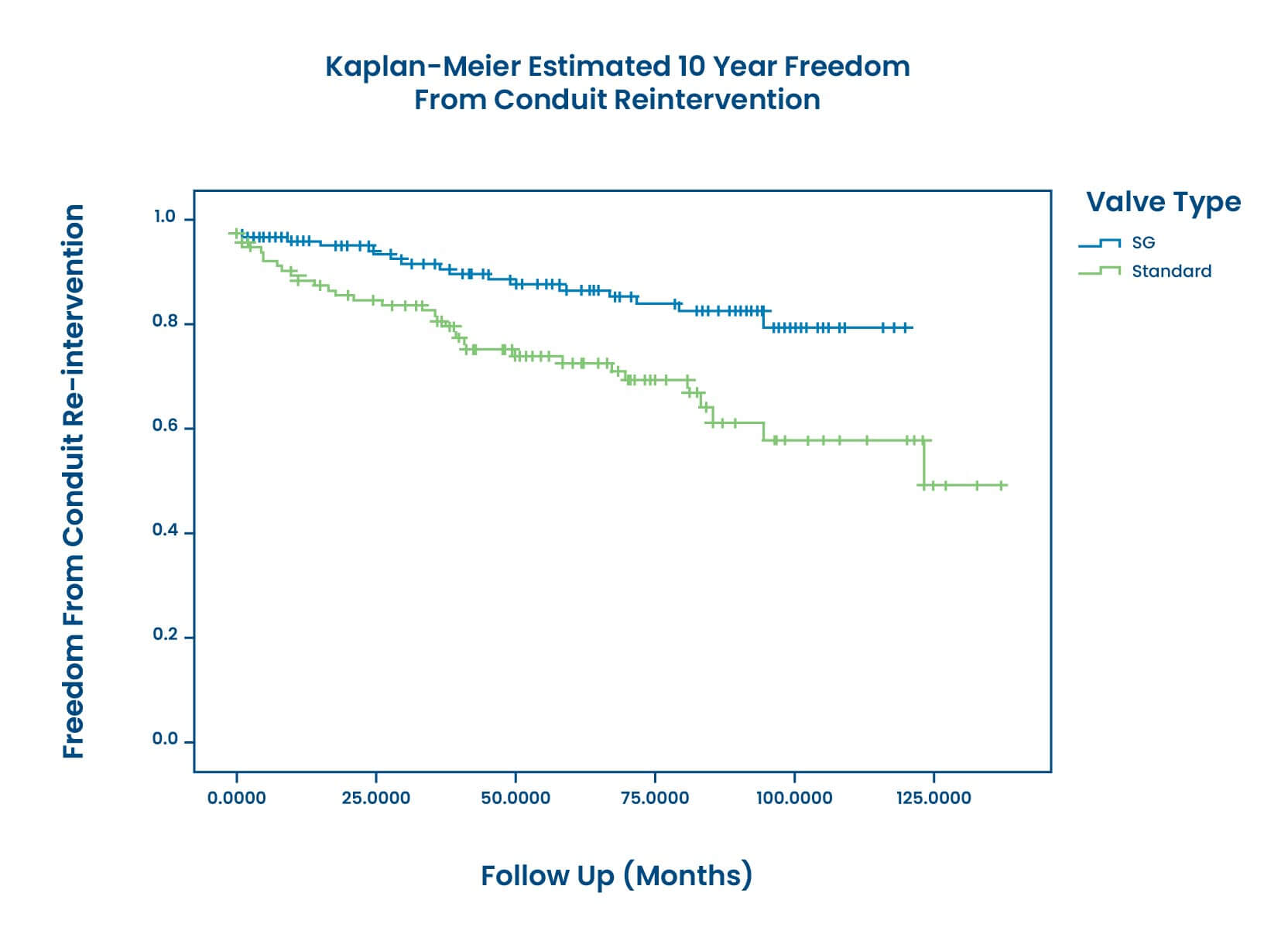
Superior Freedom from Conduit Reintervention and Dysfunction
CryoValve® SynerGraft (SG) Pulmonary Valve allografts demonstrate superior Freedom from Reintervention and Dysfunction compared to standard cryopreserved pulmonary allografts (SCA) , determined by a multi-institutional retrospective review at 10 years:1, 2
-
At 10 years, SG patients demonstrated significantly superior freedom from reintervention at a rate of 82% compared to SCA patients at 50%.1, 2
Click here to review additional Clinical Evidence for the CryoValve SG Pulmonary Valve.
Additional Resources
Explore additional resources for CryoValve SynerGraft(SG) below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Elkins R, et. al. (2001). Decellularized human valve allografts. Ann Thorac Surg, 71(5), S428-32.
- Gerson C, et. al. (2012). Structural integrity of collagen and elastin in SynerGraft decellularized-cryopreserved human heart valves. Cryobiology, 64(1), 33-42.
- Ruzmetov M, et. al. (2012). Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: A single-institution comparison. J Thorac Cardiovasc Surg, 143(3), 543-9.
- Brown JW, et. al. (2010). Performance of the CryoValve SG human decellularized pulmonary valve in 342 patients relative to the conventional CryoValve at a mean follow-up of four years. J Thorac Cardiovasc Surg, 139(2), 339-48.
- Bibevski S, et. al. (2017). Performance of SynerGraft Decellularized Pulmonary Allografts Compared With Standard Cryopreserved Allografts: Results From Multi-institutional Data. Ann Thorac Surg, 103(3), 869-75.
Product Design Features
- Elkins R, et. al. (2001). Decellularized human valve ollogrofts. Ann Thoroc Surg, 71(5), S428-32.
- Gerson C, et. al. (2012). Structural integrity of collagen and elastin in SynerGraft decellularized-cryopreserved human heart valves. Cryobiology, 64(1), 33-42.
- Artivion, Inc. Data on File.
Clinical Evidence
- Bibevski S, et. al. (2017). Performance of SynerGraft Decellularized Pulmonary Allografts Compared With Standard Cryopreserved Allografts: Results From Multi-institutional Data. Ann Thorac Surg, 103(3), 869-75.
- Fiore A. (2017) Invited Commentary. Ann Thorac Surg, 103(3), 869-75.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MWENG0061.000. (2025-05)
| Artivion, Inc., 1655 Roberts Blvd NW, Kennesaw, GA 30144, US |

