
CryoPatch® SG
PULMONARY PATCH
The Ideal Choice for Congenital Reconstruction.
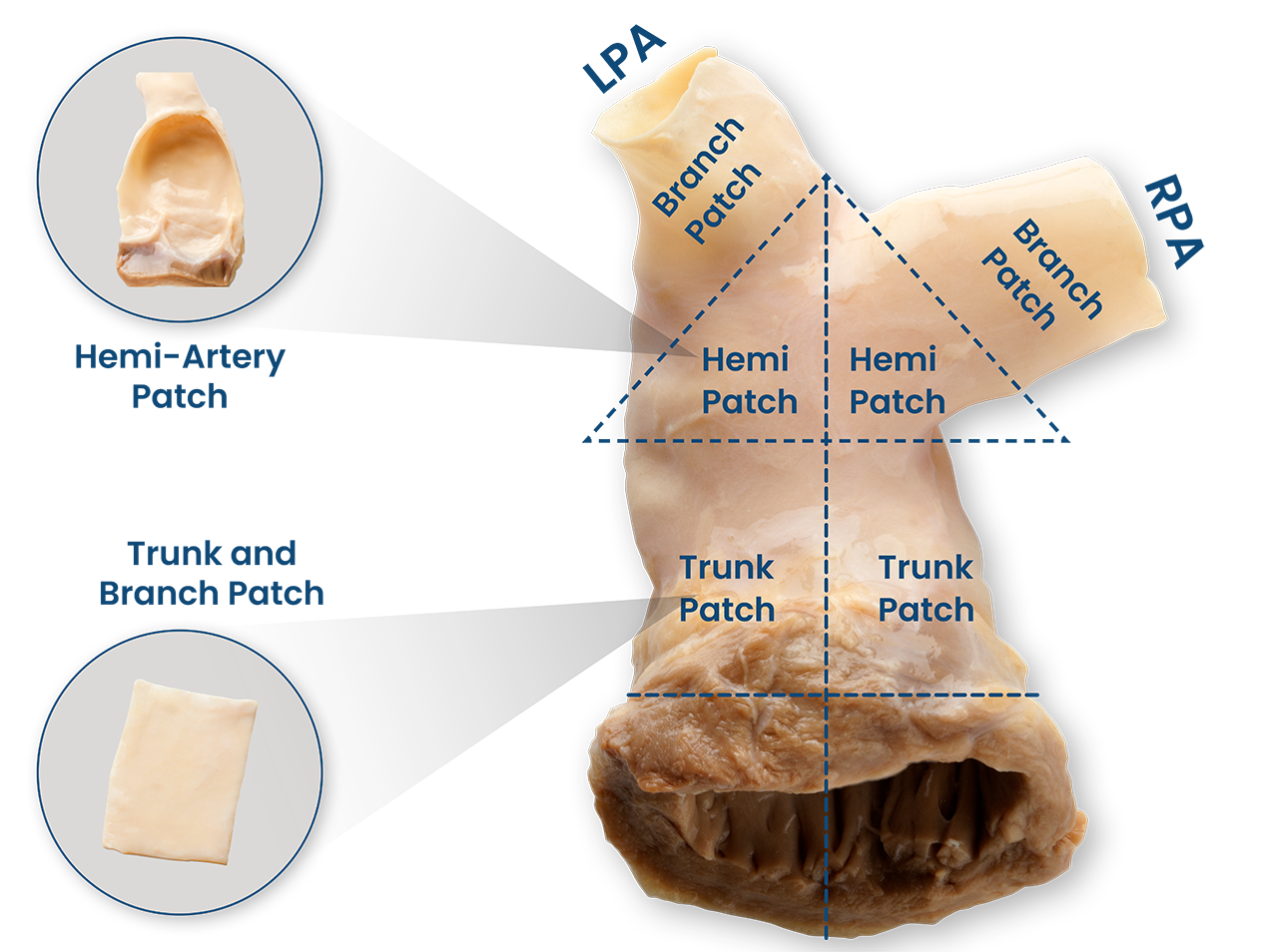
Product Highlights
-
Artivion’s SynerGraft® Technology virtually eliminates the presence of allogeneic donor cells while maintaining the structural integrity of the biological matrix.1,2
-
SynerGraft process is a detergent-free decellularization process since detergents may damage structural proteins.3-7
- Natural curvature of hemi-artery patch allows for reconstruction of single ventricle Hypoplastic Left Heart Syndrome.
Product Overview
1. SYNERGRAFT DECELLULARIZATION PROCESS
The SynerGraft process includes hypotonic cell lysis, enzymatic digestion of nucleic acids, and sequential washing in a neutral buffer. These three steps work together to ensure maximal degradation and removal of donor cell material and nucleic acids without damaging structural proteins.1-3
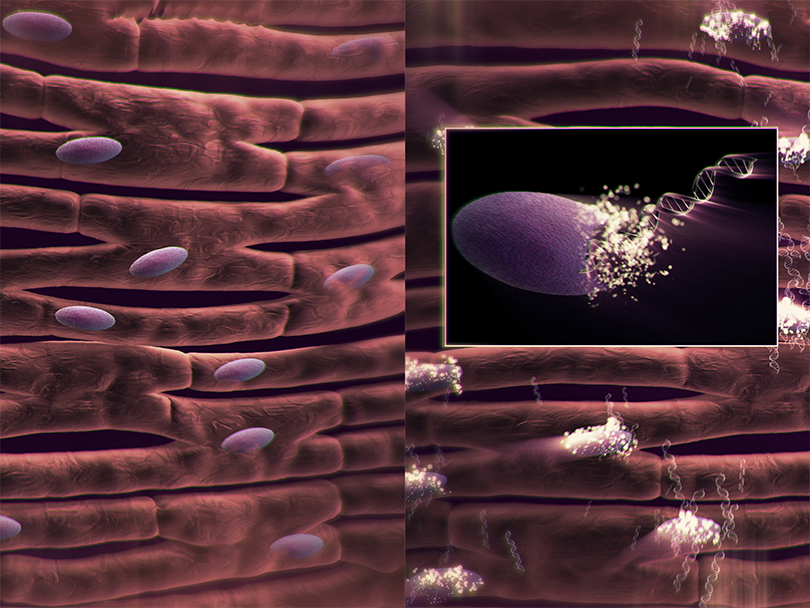
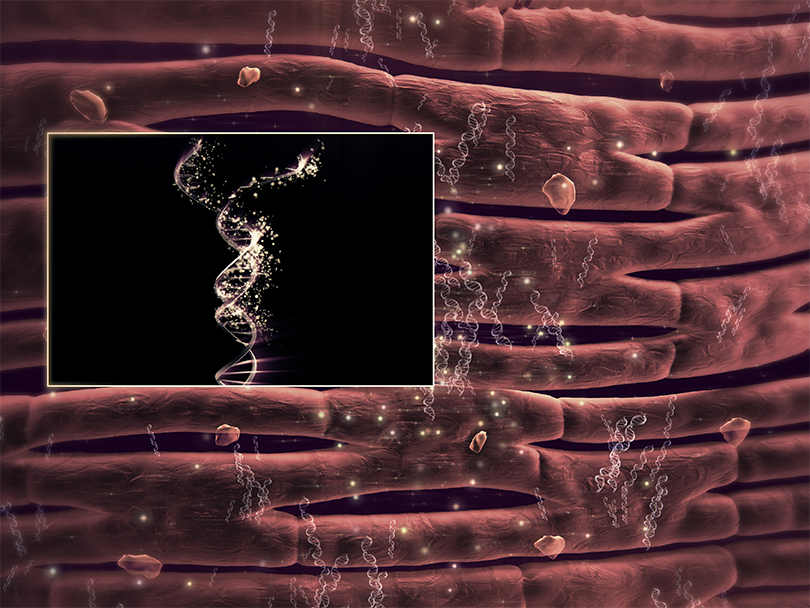
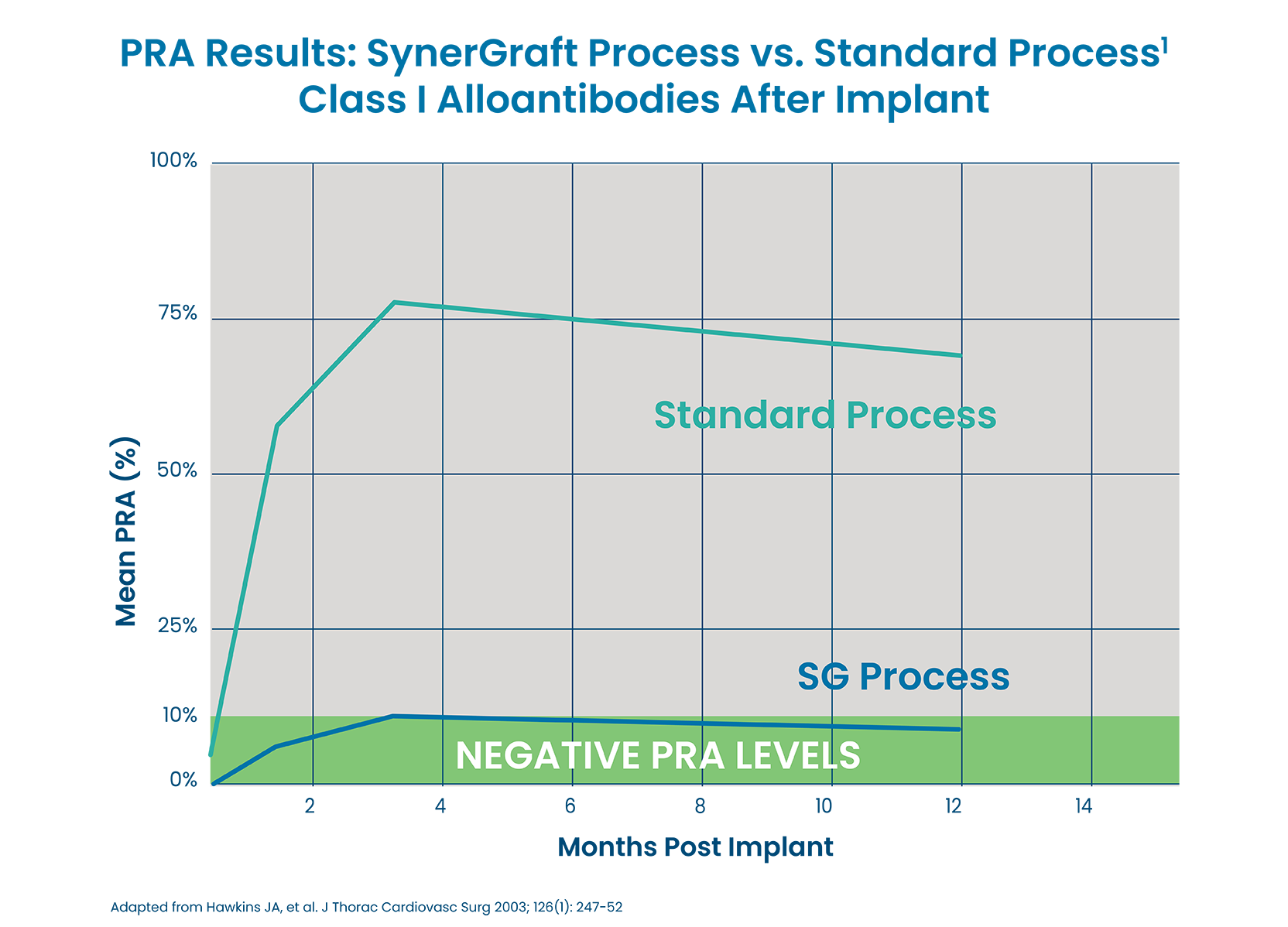
The presence of both class I and class II HLA antibodies has significant implications if future heart or other organ transplantation is needed. Implantation of decellularized grafts, with the lower level of PRA and even the absence of a response in most cases, removes this impediment to transplantation.
Clinical Evidence
SYNERGRAFT TECHNOLOGY IS PROVEN TO REDUCE PANEL REACTIVE ANTIBODIES1
Implantation of SynerGraft treated tissue reduces the risk for induction of HLA class I and class II alloantibodies based on Panel Reactive Antibody (PRA) measured at up to one year, compared to the standard processed pulmonary cardiac tissues.
Click here to read the full publication.
Additional Resources
Explore additional resources for CryoPatch below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Elkins R, et. al. (2001). Decellularized human valve allografts. Ann Thorac Surg, 71(5), S428-32.
- Gerson C, et. al. (2012). Structural integrity of collagen and elastin in SynerGraft® decellularized–cryopreserved human heart valves. Cryobiology, 64(1), 33-42.
- Zhou J, et. al. (2010). Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials, 31(9), 2549-54.
- Booth C, et. al. (2002). Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis, 11(4), 457-62.
- Korossis SA, et. al. (2002). Tissue engineering of cardiac valve prostheses II: biomechanical characterization of decellularized porcine aortic heart valves. J Heart Valve Dis, 11(4), 463–71.
- Hedman K, et. al. (1979). Isolation of the pericellular matrix of human fibroblast cultures. J Cell Biol, 81(1), 83-91.
- Yang M, et. al. (2009). Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J Biomed Mater Res Part B: Appl Biomater, 91B(1), 354-61.
Product Design Features
- Elkins R, et. al. (2001). Decellularized human valve allografts. Ann Thorac Surg, 71(5), S428-32.
- Gerson C, et. al. (2012). Structural integrity of collagen and elastin in SynerGraft® decellularized–cryopreserved human heart valves. Cryobiology, 64(1), 33-42.
- Artivion, Inc. Data on file.
Clinical Evidence
- Hawkins JA, et. al. (2003). Immunogenicity of decellularized cryopreserved allografts in pediatric cardiac surgery: comparison with standard cryopreserved allografts. J Thorac Cardiovasc Surg, 126(1), 247-52.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1592.000. (2023-04)
| Artivion, Inc. 1655 Roberts Blvd, Kennesaw, GA 30144, US |

