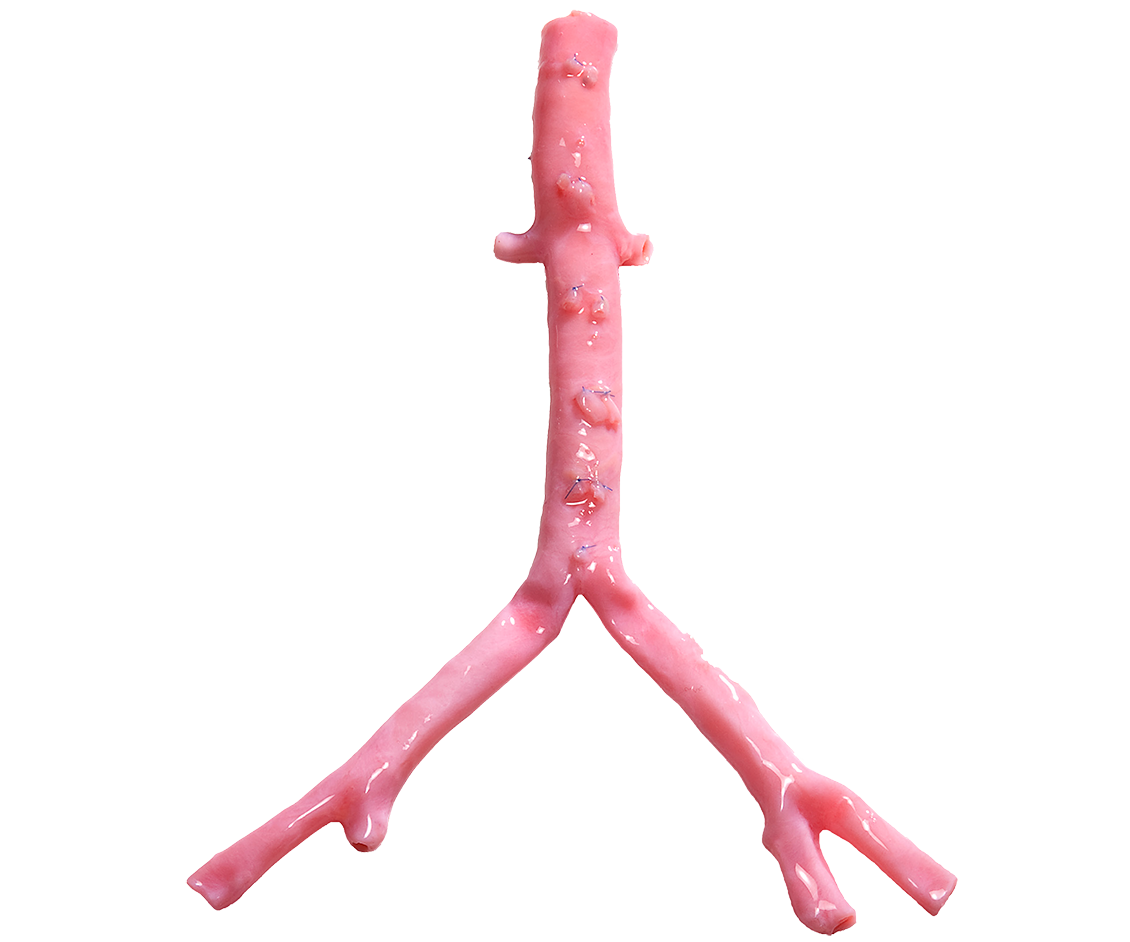
CryoArtery®
AORTOILIAC ARTERY
The Natural Choice
for Infected Fields.
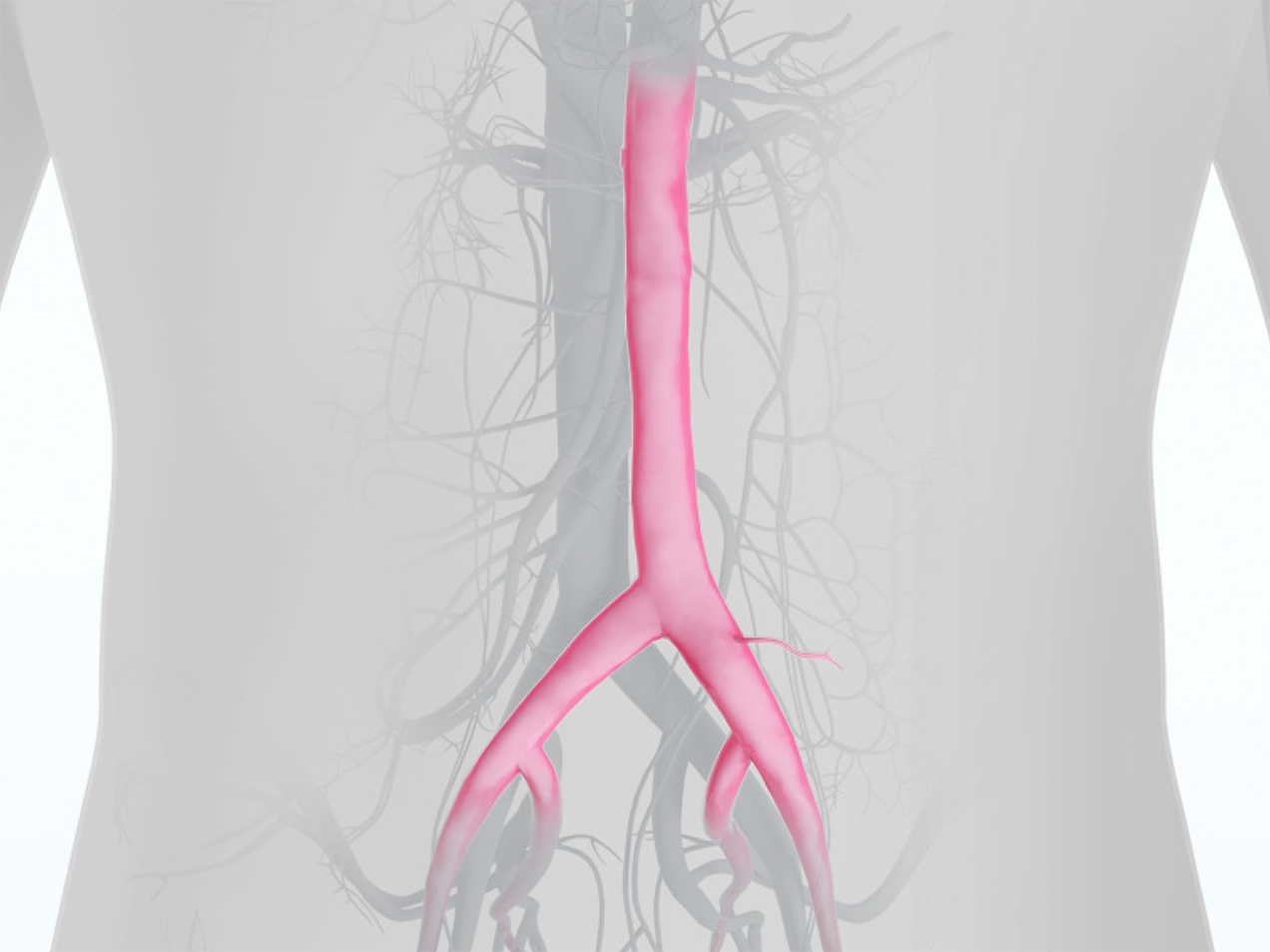
Product Highlights
- The ideal solution for treating infected fields and replacing infected synthetic grafts.1-5
- Outstanding structural durability with no reports of aneurysm or dilation in four U.S. studies.2-5
- Potential time and cost savings in the operating room compared with alternative procedures.1,4,6-10
Product Overview
1. Superior Quality
Artivion significantly exceeds FDA and AATB donor rejection criteria standards to benefit patient care and outcomes.1,2 Strict criteria standards and Artivion’s proprietary tissue processing provide superior quality allografts
2. Excellent Clinical Performance
The CryoArtery Aortoiliac Artery demonstrates 96% freedom from reinfection and 97% patency at 5 years.3
Our long-term results are excellent, with up to 10-year follow-ups in some patients.
Clinical Evidence
The Natural Choice for Infected Fields
CryoArtery Aortoiliac Artery is the ideal graft for replacing infected synthetic aortic grafts and for patients at high risk of infection.1-5
- 96% Freedom from Re-infection at 5-Years.6
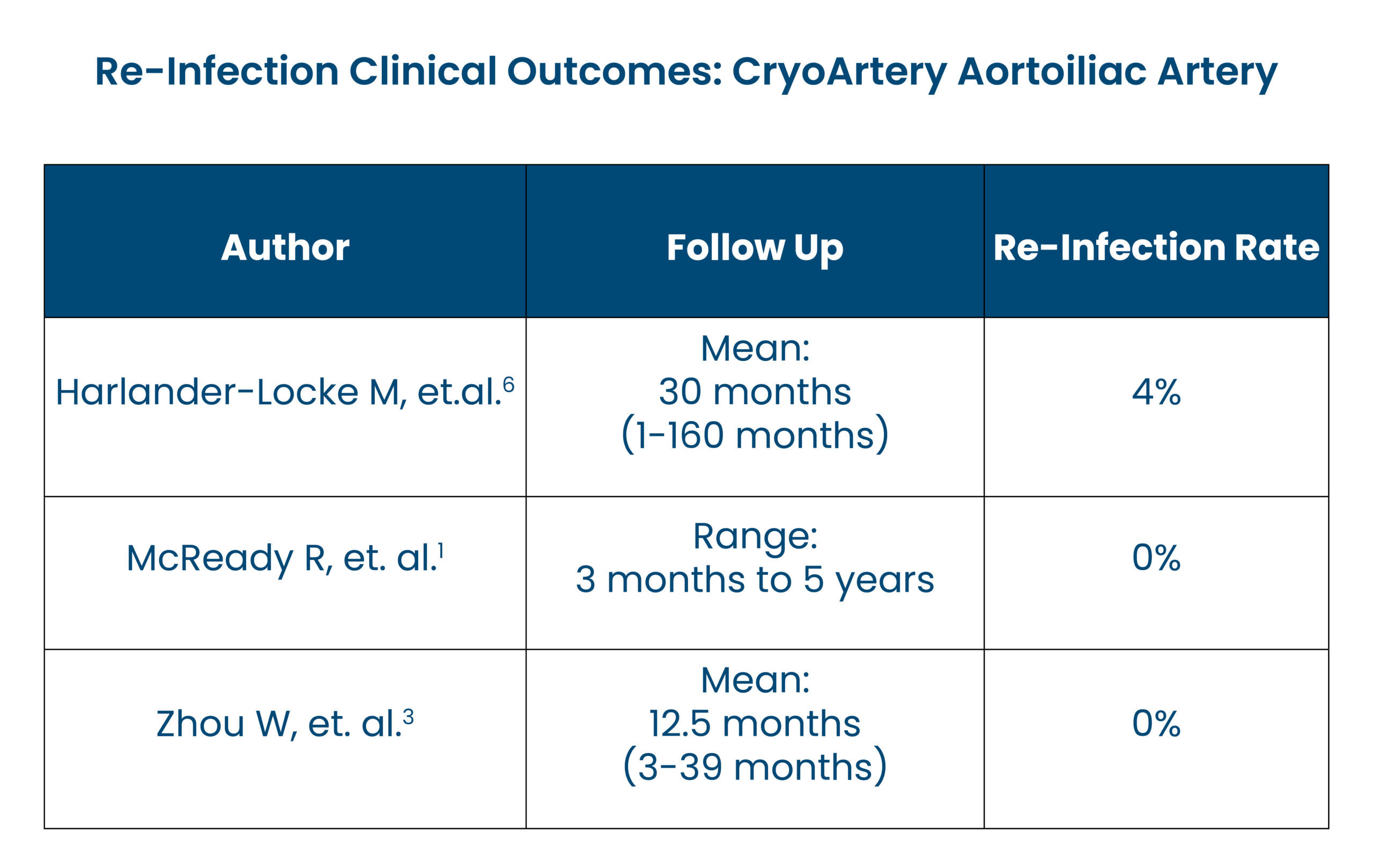
The use of cryopreserved aortoiliac allograft for aortic reconstruction in the United States Publication
Additional Resources
Explore additional resources for CryoArtery below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Harlander-Locke M, et. al. (2014). The use of cryopreserved aortoiliac allograft for aortic reconstruction in the United States. J Vasc Surg, 59(3), 669-74.
- McCready R, et. al. (2006). Arterial Infections in the New Millennium: An Old Problem Revisited. Ann Vasc Surg, 20(5), 590-5.
- Noel AA, et. al. (2002). United States Cryopreserved Aortic Allograft Registry. Abdominal aortic reconstruction in infected fields: early results of the United States cryopreserved aortic allograft registry. J Vasc Surg, 35(5), 847-52.
- Zhou W, et. al. (2006). In Situ Reconstruction with Cryopreserved Arterial Allografts for Management of Mycotic Aneurysms or Aortic Prosthetic Graft Infections: A Multi-Institutional Experience We designed this study to evaluate a multi-institutional experience. Tex Heart Inst J, 33(1), 14-18.
- Brown KE, et. al. (2009). Arterial reconstruction with cryopreserved human allografts in the setting of infection: A single-center experience with midterm follow-up. J Vasc Surg, 49(3), 660-6.
- Vardanian A, et. al. (2009). Arterial Allograft Allows In-line Reconstruction of Prosthetic Graft Infection with Low Recurrence Rate and Mortality. Am Surg, 75(10), 1000-3.
- Ali AT, et. al. (2009). Long-term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts. J Vasc Surg, 50(1), 30-9.
- Liedenbaum MH, et. al. (2009). The Outcome of the Axillofemoral Bypass: A Retrospective Analysis of 45 Patients. Worl J Surg, 33(11), 2490-6.
- Bandyk DF, et. al. (2001). Use of rifampin-soaked gelatin-sealed polyester grafts for in situ treatment of primary aortic and vascular prosthetic infections. J Surg Res, 95(1), 44-9.
- Vogt P, et. al. (1998). Cryopreserved arterial allografts in the treatment of major vascular infection: A comparison with conventional surgical techniques, J Thorac Cardiovasc Surg, 116(6), 965-72.
Product Overview
- The American Association of Tissue Banks Standards for Tissue Banking (Current Edition). https://www.aatb.org/standards. Accessed on February 9, 2023. Criteria reviewed at time of donation.
- FDA Donor Eligibility Regulations 21 CFR part 1271, Subpart C. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1271/subpart-C?toc=1. Accessed on February 9, 2023.
- Harlander-Locke M, et. al. (2014). The use of cryopreserved aortoiliac allograft for aortic reconstruction in the United States. J Vasc Surg, 59(3), 669-74.
Clinical Evidence
- McCready R, et. al. (2006). Arterial Infections in the New Millennium: An Old Problem Revisited. Ann Vasc Surg, 20(5), 590-5.
- Noel AA, et. al. (2002). United States Cryopreserved Aortic Allograft Registry. Abdominal aortic reconstruction in infected fields: early results of the United States cryopreserved aortic allograft registry. J Vasc Surg, 35(5), 847-52.
- Zhou W, et. al. (2006). In Situ Reconstruction with Cryopreserved Arterial Allografts for Management of Mycotic Aneurysms or Aortic Prosthetic Graft Infections: A Multi-Institutional Experience We designed this study to evaluate a multi-institutional experience. Tex Heart Inst J, 33(1), 14-18.
- Vogt P, et. al. (1998). Cryopreserved arterial allografts in the treatment of major vascular infection: A comparison with conventional surgical techniques, J Thorac Cardiovasc Surg, 116(6), 965-72.
- Brown KE, et. al. (2009). Arterial reconstruction with cryopreserved human allografts in the setting of infection: A single-center experience with midterm follow-up. J Vasc Surg, 49(3), 660-6.
- Harlander-Locke M, et. al. (2014). The use of cryopreserved aortoiliac allograft for aortic reconstruction in the United States. J Vasc Surg, 59(3), 669-74.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1588.000. (2023-04)
| Artivion, Inc. 1655 Roberts Blvd, Kennesaw, GA 30144, US |

