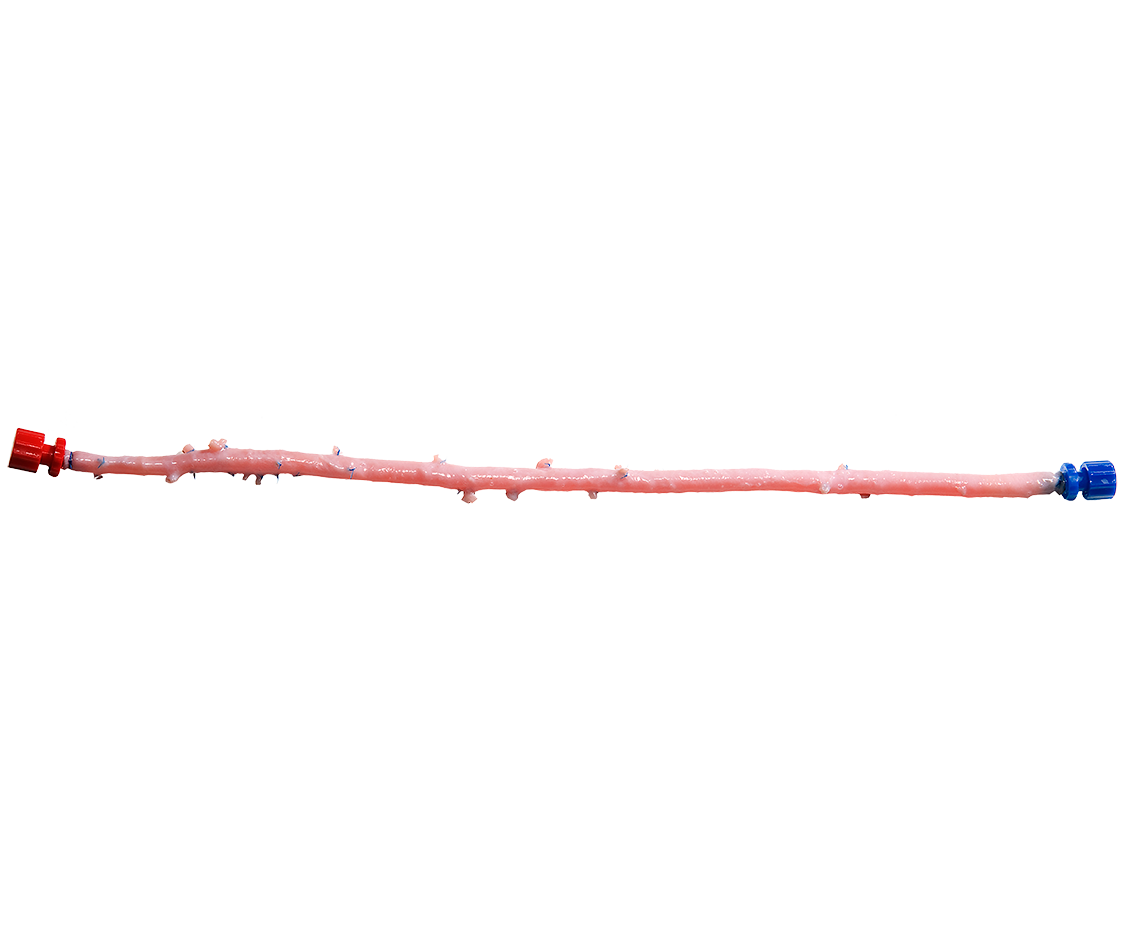
CryoArtery®
FEMORAL ARTERY
The Natural Choice for Vascular Reconstruction.
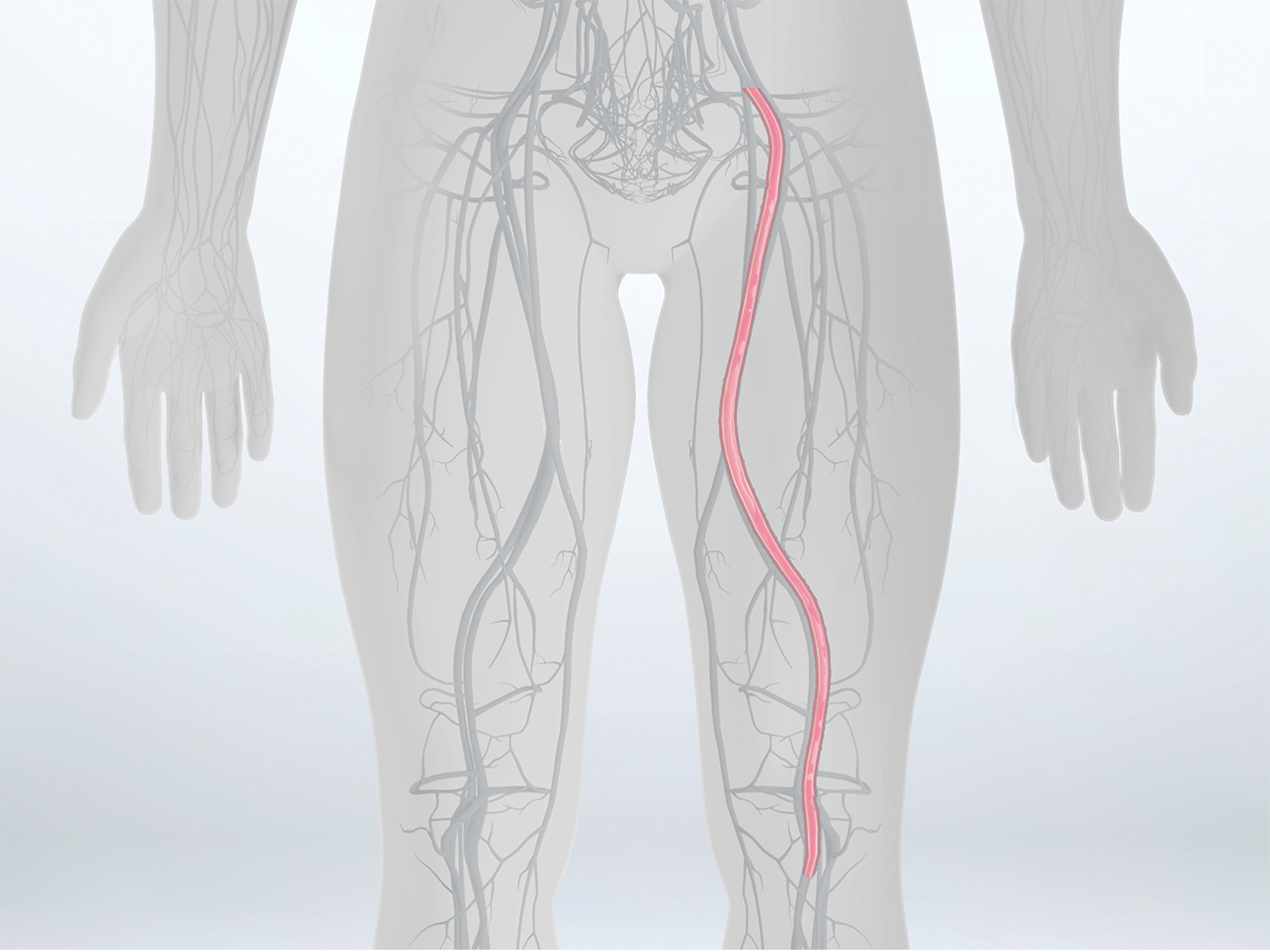
Product Highlights
- Excellent resistance to infection and the ideal solution for patients at risk of infection.1
- High limb salvage rates for bypass as up to 30% of patients have no suitable autologous tissue.1-4
- Natural suturability and natural pulsatile flow to treat patients with Critical Limb Ischemia.5,6
Product Overview
1. Superior Quality
Artivion significantly exceeds FDA and AATB donor rejection criteria standards to benefit patient care and outcomes.1,2 Strict criteria standards and Artivion’s proprietary tissue processing provide superior quality allografts.
2. Excellent Clinical Performance
The CryoArtery Femoral Artery demonstrates 100% freedom from infection at 3 years.3
Clinical Evidence
The Natural Choice for Vascular Reconstruction
CryoArtery Femoral Artery is the ideal graft for below-knee revascularization in patients with Critical Limb Ischemia (CLI), patients at risk of infection, or those without suitable autologous tissue for bypass.1
- 100% Freedom from Infection at 3-Years2
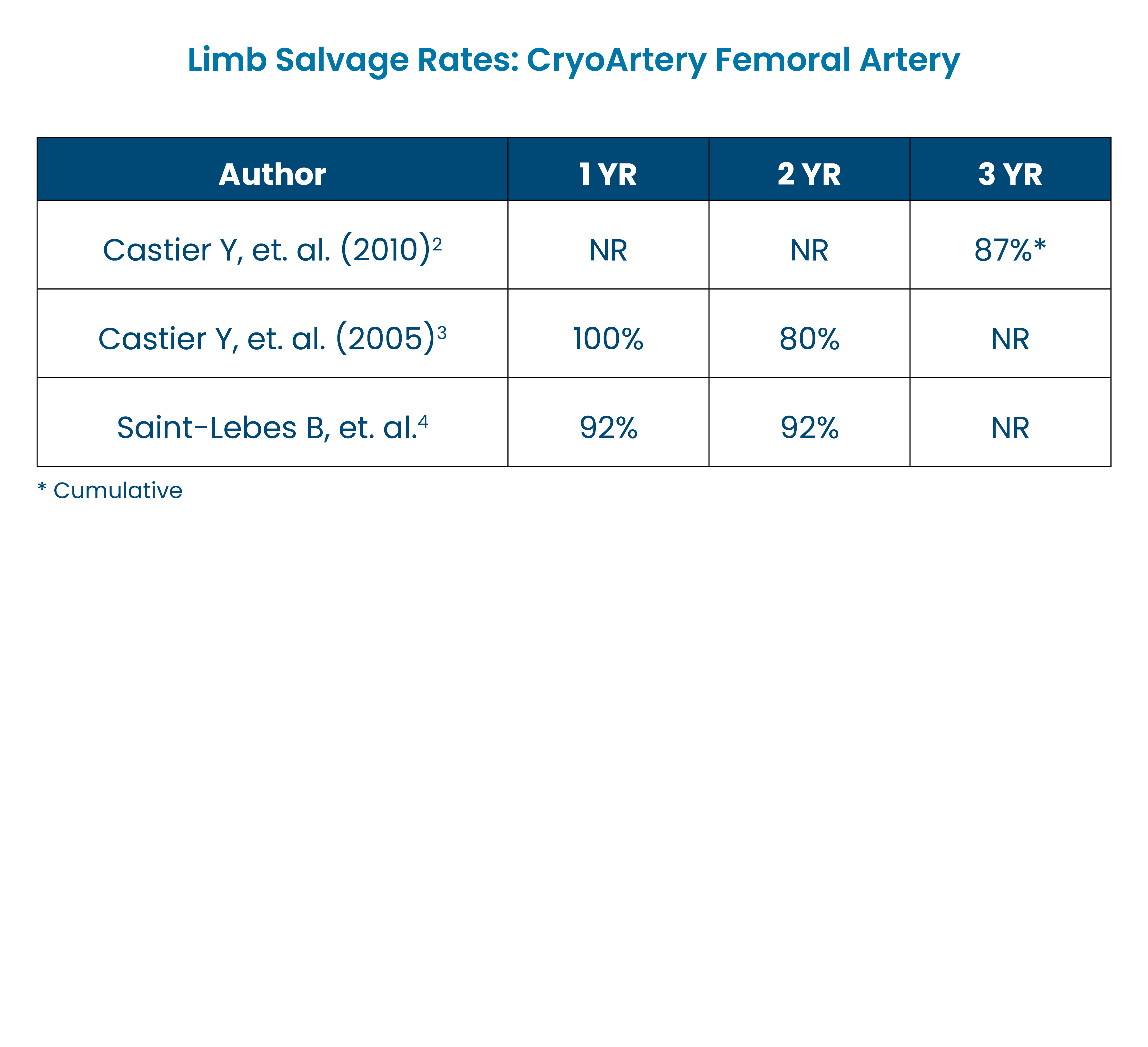
- High Limb Salvage Rates Across Multiple Studies2-4
Additional Resources
Explore additional resources for CryoArtery below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Castier Y, et. al. (2005). Cryopreserved arterial allograft reconstruction for peripheral graft infection. J Vasc Surg, 41, 30-7.
- Randon C, et. al. (2010). Fifteen years of infrapopliteal arterial reconstructions with cryopreserved venous allografts for limb salvage. J Vasc Surg, 51(4), 869-77.
- Castier Y, et. al. (2010). Cryopreserved Arterial Allograft Reconstruction for Infected Peripheral Bypass. Ann Vasc Surg, 24(8), 994-9.
- Saint-Lebes B, et. al. (2015). Cryopreserved Arterial Allografts for the Revascularization of Patients Suffering from Critical Ischemia of the Lower Extremities in the absence of Vein. Prospective and Multicentric Study. Ann Vasc Surg, 29(6), 1068.
- Martin RS, et. al. (1994). Cryopreserved Saphenous Vein Allografts for Below-Knee Lower Extremity Revascularization. Ann Vasc Surg, 219(6), 664-72.
- Bia D, et. al. (2007). Differential functional coupling between human saphenous cryoallografts and arteries: Importance of the arterial type and the biomechanical parameter evaluated. Artificial Organs, 31(11), 809–18.
Product Design Features
- The American Association of Tissue Banks Standards for Tissue Banking (Current Edition). https://www.aatb.org/standards. Accessed on February 9, 2023. Criteria reviewed at time of donation.
- FDA Donor Eligibility Regulations 21 CFR part 1271, Subpart C. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1271/subpart-C?toc=1. Accessed on February 9, 2023.
- Castier Y, et. al. (2005). Cryopreserved arterial allograft reconstruction for peripheral graft infection. J Vasc Surg, 41, 30-7.
Clinical Evidence
- Randon C, et. al. (2010). Fifteen years of infrapopliteal arterial reconstructions with cryopreserved venous allografts for limb salvage. J Vasc Surg, 51(4), 869-77.
- Castier Y, et. al. (2010). Cryopreserved Arterial Allograft Reconstruction for Infected Peripheral Bypass. Ann Vasc Surg, 24(8), 994-9.
- Castier Y, et. al. (2005). Cryopreserved arterial allograft reconstruction for peripheral graft infection. J Vasc Surg, 41, 30-7.
- Saint-Lebes B, et. al. (2015). Cryopreserved Arterial Allografts for the Revascularization of Patients Suffering from Critical Ischemia of the Lower Extremities in the absence of Vein. Prospective and Multicentric Study. Ann Vasc Surg, 29(6), 1068.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1599.000. (2023-04)
| Artivion, Inc., 1655 Roberts Blvd NW, Kennesaw, GA 30144, US |

