
CarbonAid® & CarbonMini®
CO2 DIFFUSERS
Reduce Risk of Air Embolism During Open Heart Surgery.
Product Highlights
- Reduces risk of air embolism by more effectively de-airing.1,*
- Significantly reduces the time for microemboli to disappear.2
- May prevent infections by creating a >99% bacteriostatic atmosphere.3,4
Product Overview
1. DIFFUSER TIP
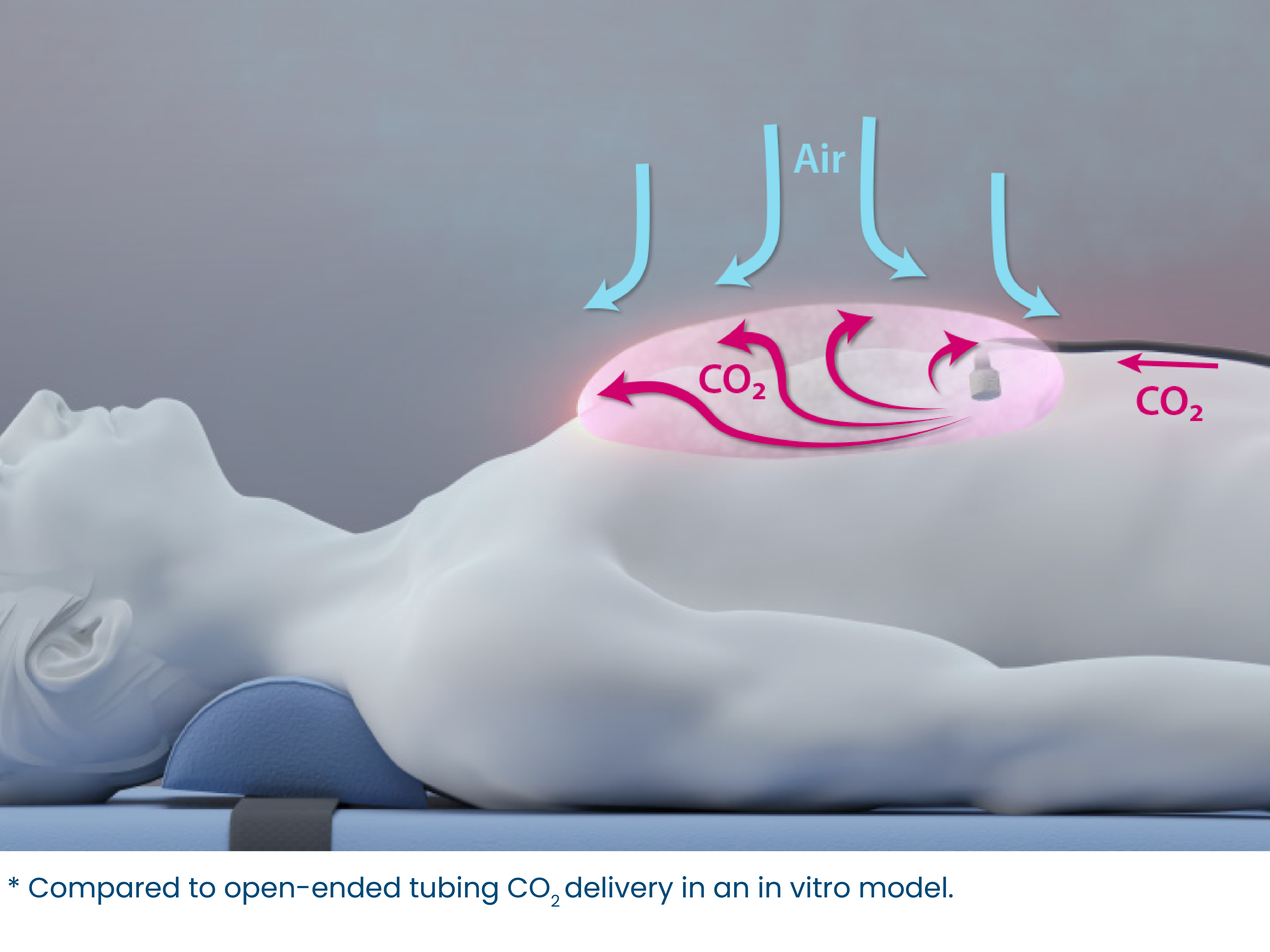
Foam tip allows CO2 to be delivered in a high flow, but diffused in a low velocity manner, allowing CO2 to fully fill the surgical wound cavity during open heart procedures by replacing air with > 99% CO2.
2. STAR SHAPED TUBING
Star shaped, wire reinforced tubing allows CO2 to flow freely, even while bent for easy and secure surgical positioning in the wound cavity.

3. PVC tubing with adequate length for connection to tank or central line CO2

1. Star shaped Tubing
CarbonAid unique star shaped inner tubing, allows unobstructed, free flowing CO2 while bending tubing for easy surgical cavity positioning.
2. Reinforced Tubing
Bendable reinforced wire tubing near the tip end allows the foam tip to be securely positioned into place in the wound cavity.
3. Star shaped wire
Allows free flowing CO2 while bending tubing for easy surgical cavity positioning. Bendable reinforced wire tubing near the foam tip end allows the foam tip to be securely positioned into place in the wound cavity.

A lot of the time is spent manipulating the heart, de-airing, and evaluating with the echocardiogram when you have air that you have to get rid of. I think that CarbonAid reduces time significantly. When you see the particles disperse and disappear over a period of minutes as opposed to 10 or 15 minutes, I think you can safely say that’s a reduction in the time that you are on bypass. If you don’t think it’s significant, talk to some people, and you will see that 15 minutes bypass time or whatever additional time that you spend can have pretty adverse effects on patients.
Clinical Evidence
Demonstrated Superiority to Other Diffusors
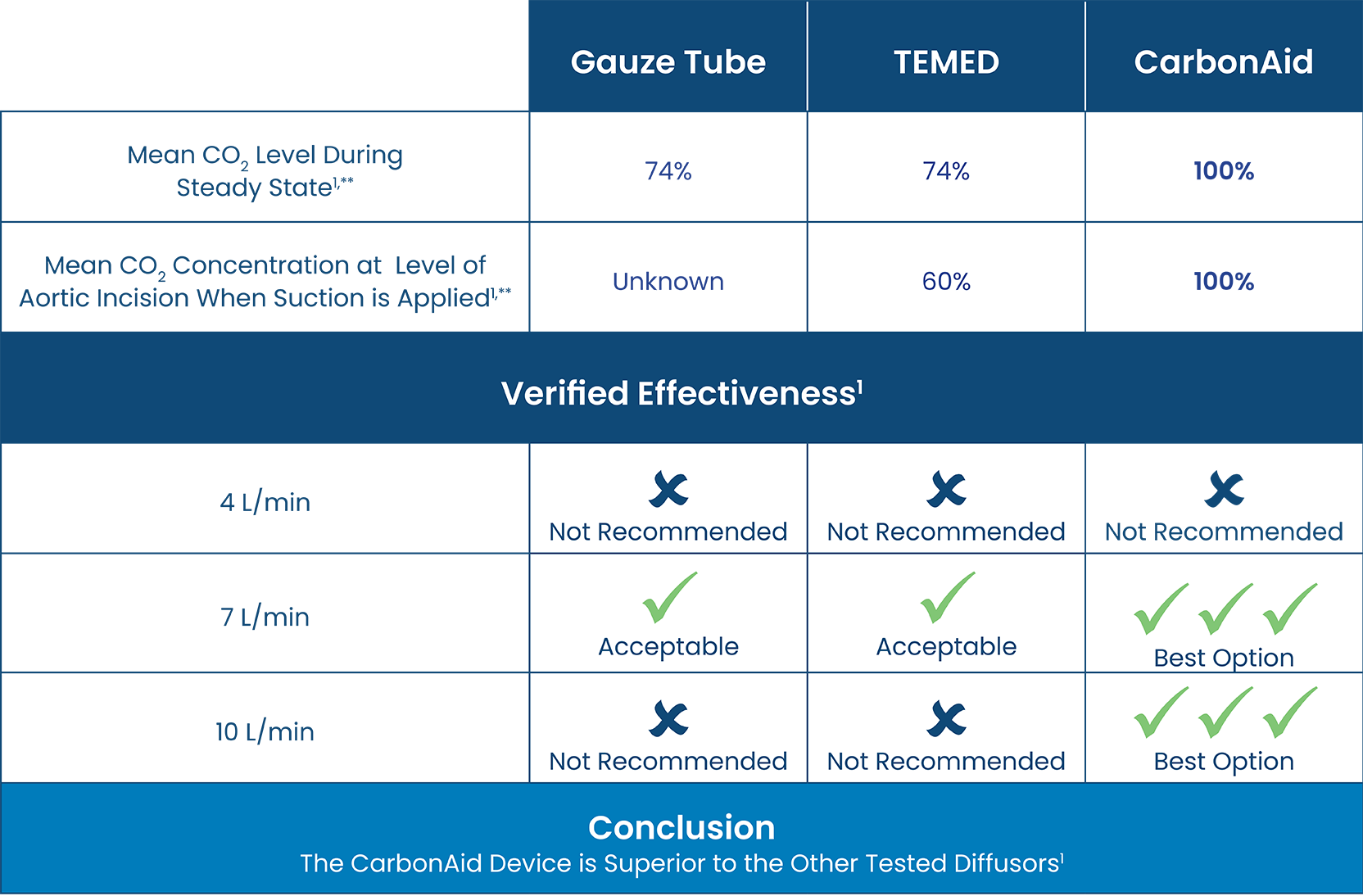
A robust, independent clinical study comparing CO2 field flooding devices, including CarbonAid and other commercial and improvised deairing devices, concluded that the CarbonAid device is superior to other devices or methods.1,*,**
*Comparison of CO2 delivery options from in-vitro experiment data.
** When measured at 10L/minute.
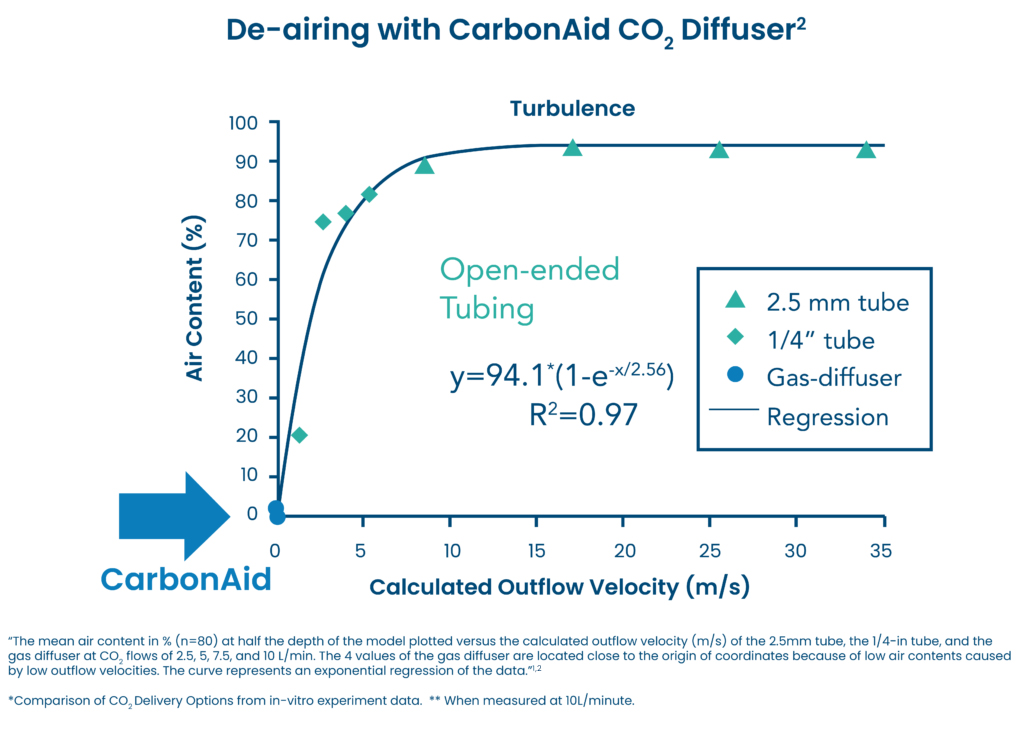
CarbonAid CO2 Diffusers Provide Near Complete De-Airing
CarbonAid Deairing CO2 Diffuser provided an almost complete de-airing of the model (<0.2% remaining air) at flows of 5-10 L/min. This was the result of a uniform distribution of CO2 with a calculated velocity of about 0.1 meter/second.2
Additional Resources
Explore additional resources for CarbonAid below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- Persson M. et al. (2003) De-airing of a Cardiothoracic Wound Cavity Model With Carbon Dioxide: Theory and Comparison of a Gas Diffuser With Conventional Tubes. J Cardiothorac Vasc Anesth. 17(3):329-35.
- Svenarud P. et al. (2004) Effect of CO2 Insufflation on the Number and Behavior of Air Microemboli in Open-Heart Surgery. A Randomized Clinical Trial. Circ. 109(9):1127-32.
- Persson M. et al. (2005) Carbon dioxide inhibits the growth rate of Staphylococcus aureus at body temperature. Surg Endosc.19(1):91-4.
- Persson M. et al. (2004) Wound ventilation with carbon dioxide: a simple method to prevent direct airborne contamination during cardiac surgery? J Hosp Infect. 56(2):131-6.
Clinical Evidence
- Vandenberghe S. et. al. (2020) Direct Visualization of Carbon dioxide field flooding: Optical and concentration level comparison of diffusor effectivness. J Thorac Cardiovasc Surg. 159(3):958-968. doi: 10.1016/j.jtcvs.2019.04.040.
- Persson M. et al. (2003) De-airing of a Cardiothoracic Wound Cavity Model With Carbon Dioxide: Theory and Comparison of a Gas Diffuser With Conventional Tubes. J Cardiothorac Vasc Anesth. 17(3):329-35.
CarbonAid and CarbonMini are distributed by Artivion, Inc. The CarbonAid and CarbonMini products are manufactured by Cardia Innovation AB. CarbonAid and CarbonMini are registered trademarks of Cardia Innovation AB. All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc.
MLENG1609.000. (2023-04)
|
Cardia Innovation AB | Månskärsvägen 10 B | 141 75 Kungens Kurva, Sweden |

