Artivex™
THORACIC EXTENSION STENT GRAFT SYSTEM
CUSTOM-MADE DEVICE
Extend More.
Product Highlights
- Engineered for a stable and accurate connection
- Strong taper up to 20 mm
- Impressive longitudinal stability
- Dedicated cylindrical landing zones
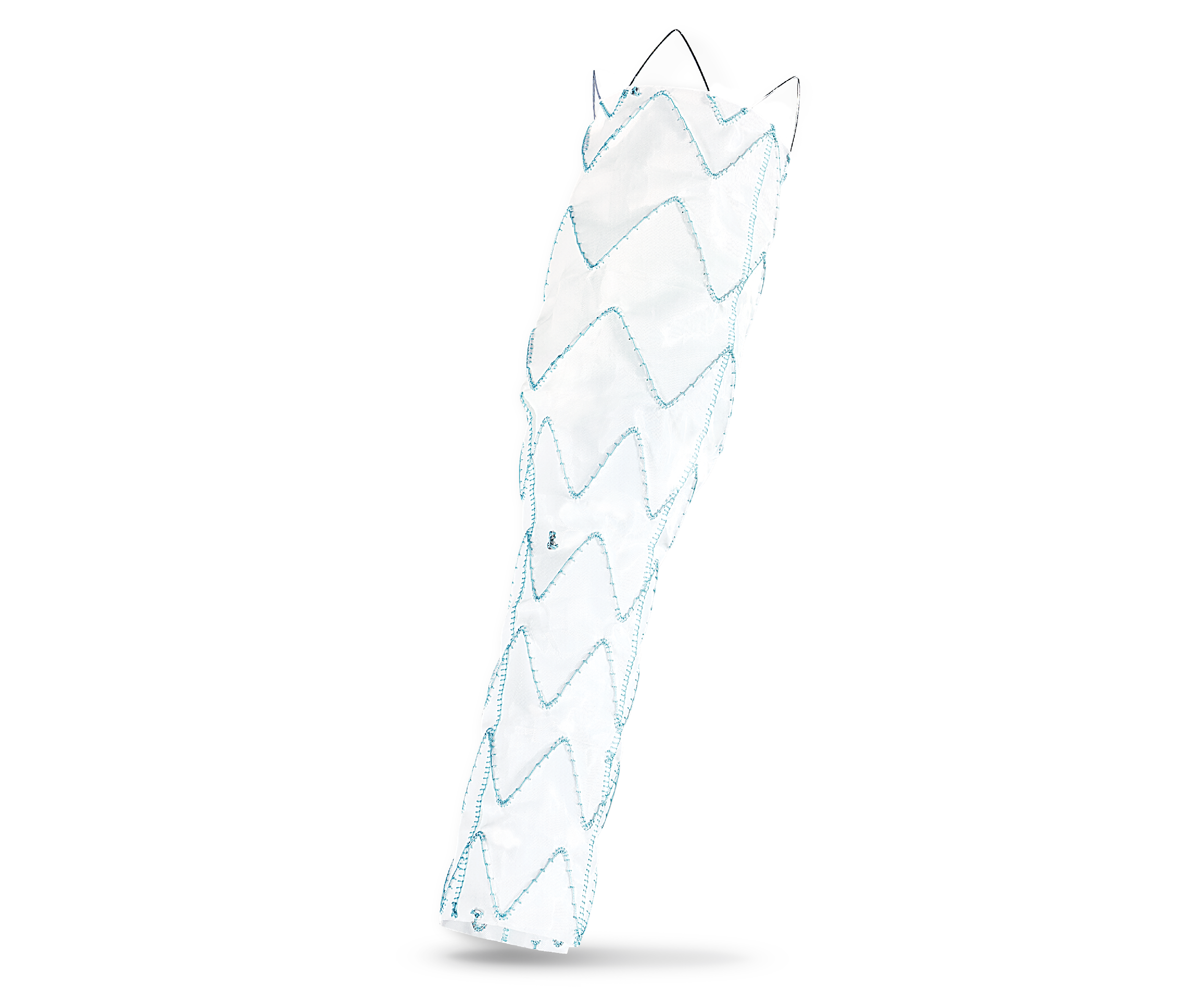
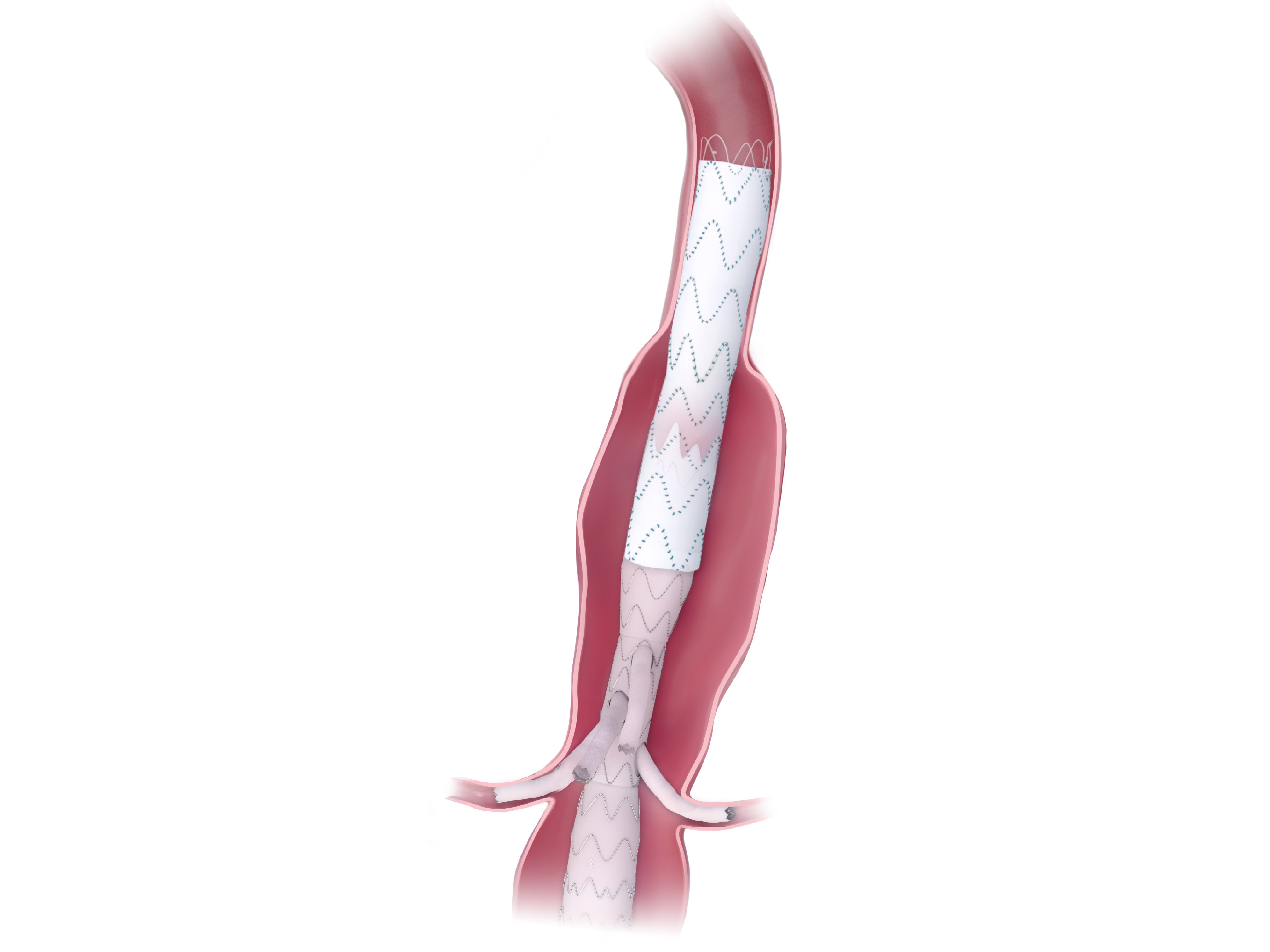
Product Overview
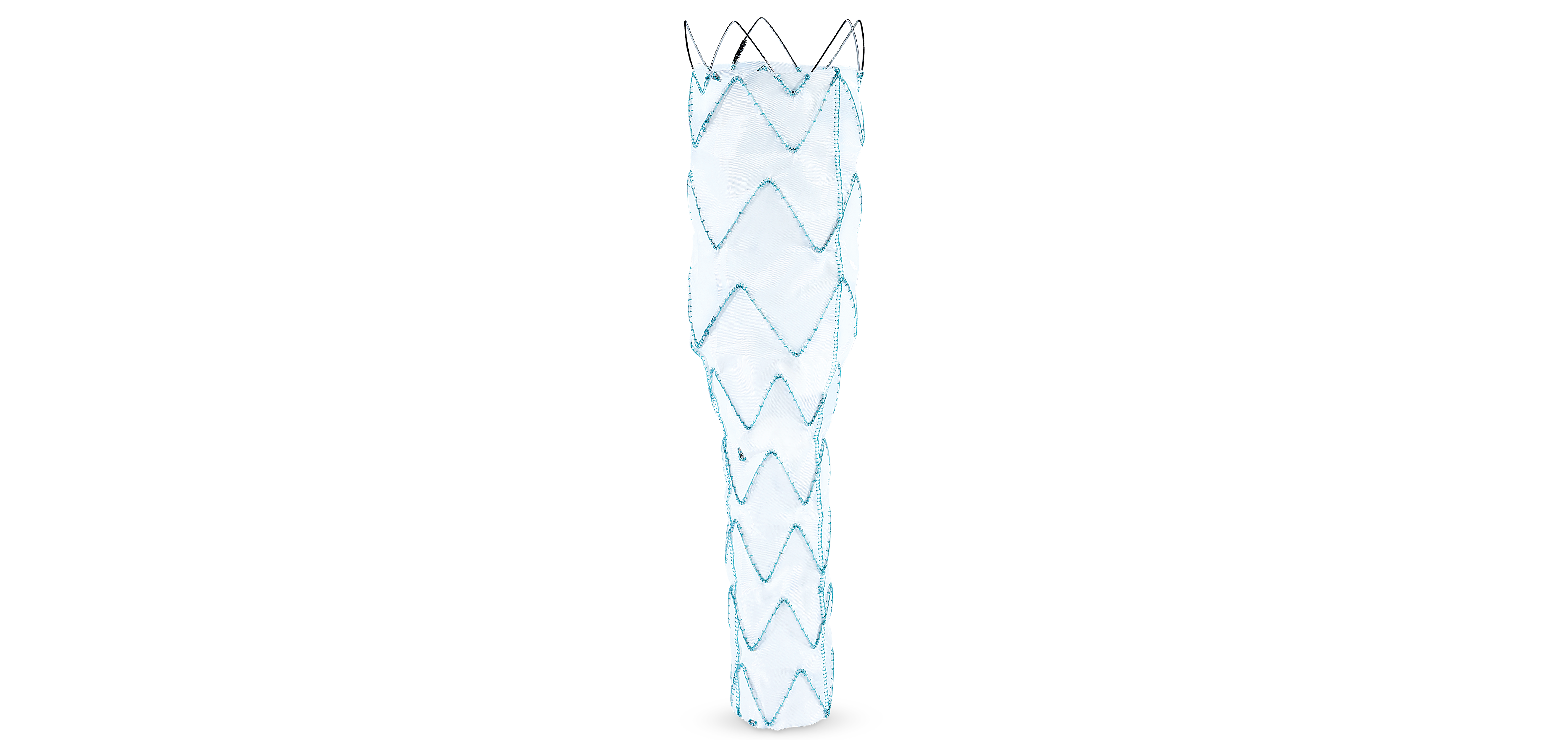
1. ARTIVEX PROXIMAL DESIGN
Straight Open
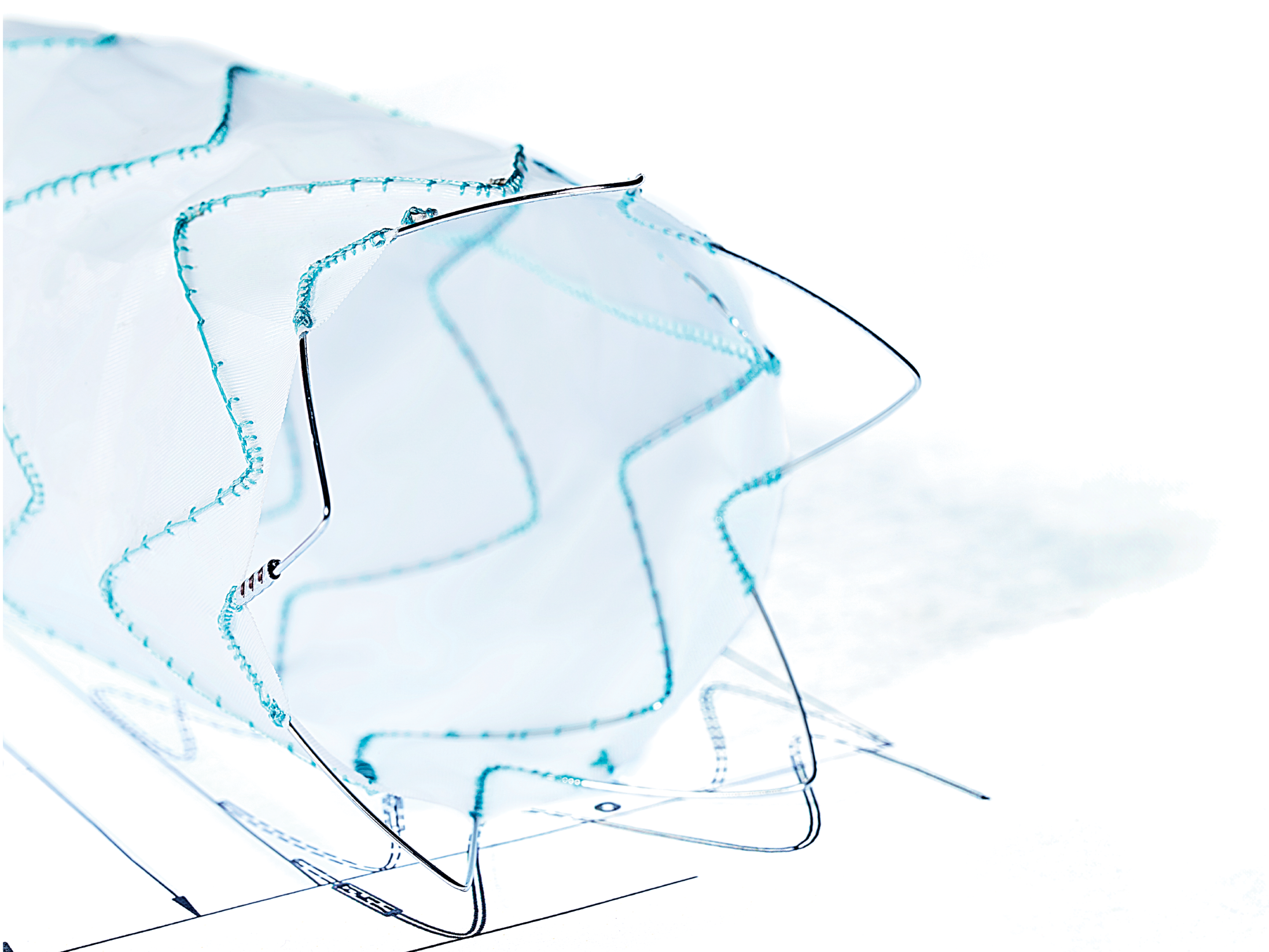
2. ARTIVEX MARKERS
For clear visibility, Artivex has platinum-iridium X-ray markers. Two ring markers define the proximal end of the implant fabric.
3. ARTIVEX STENT DESIGN
Each spring is made out of etched and electropolished nitinol
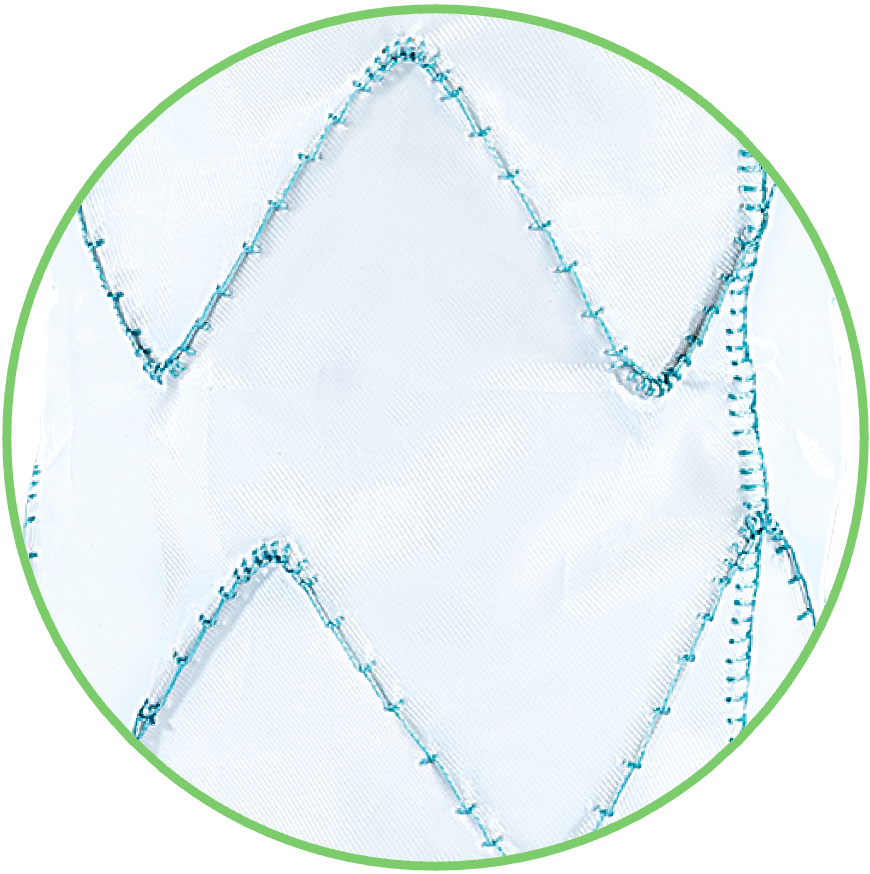
4. ARTIVEX IMPLANT FABRIC
Is made out of woven polyester with low water permeability.
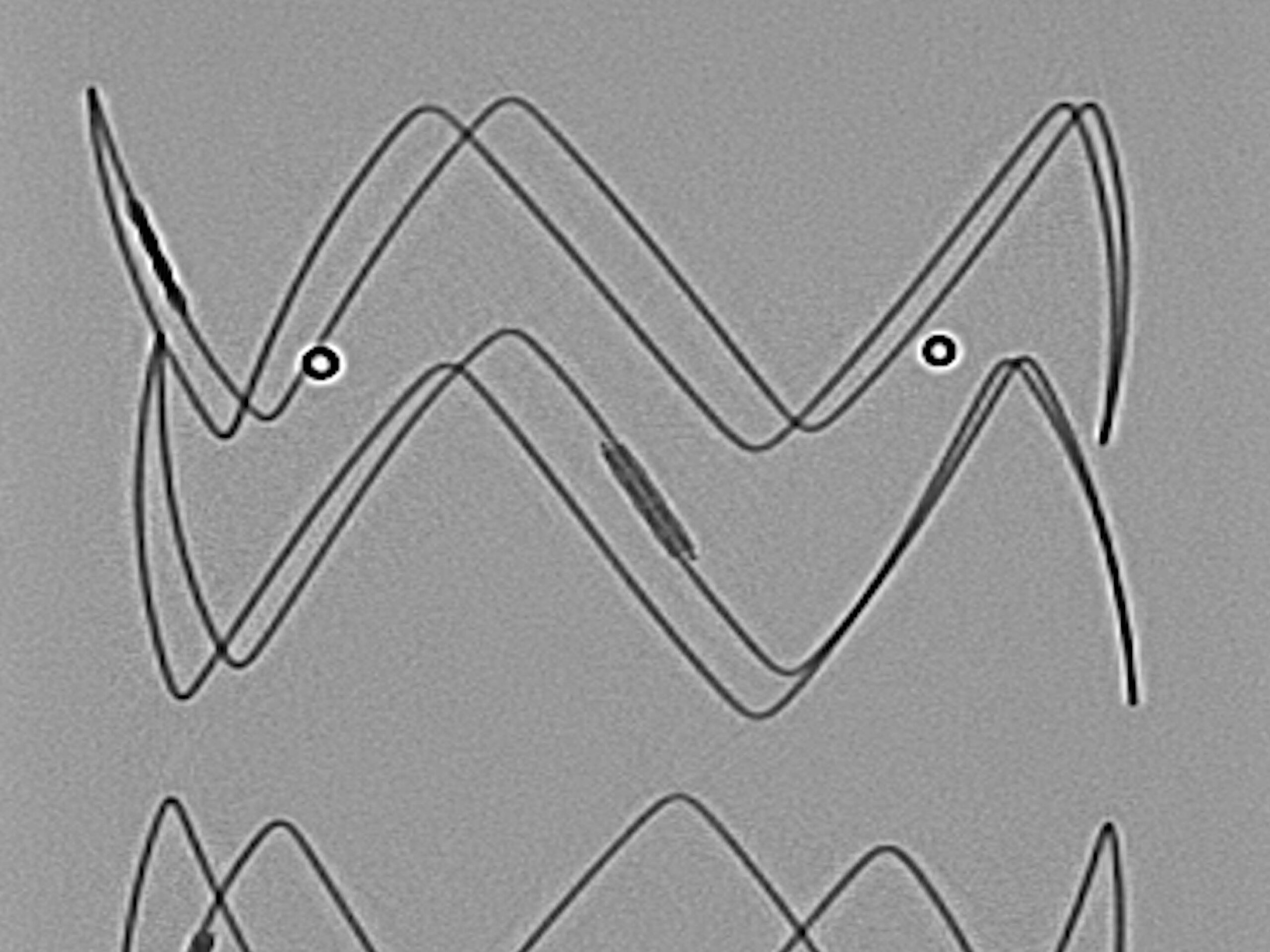
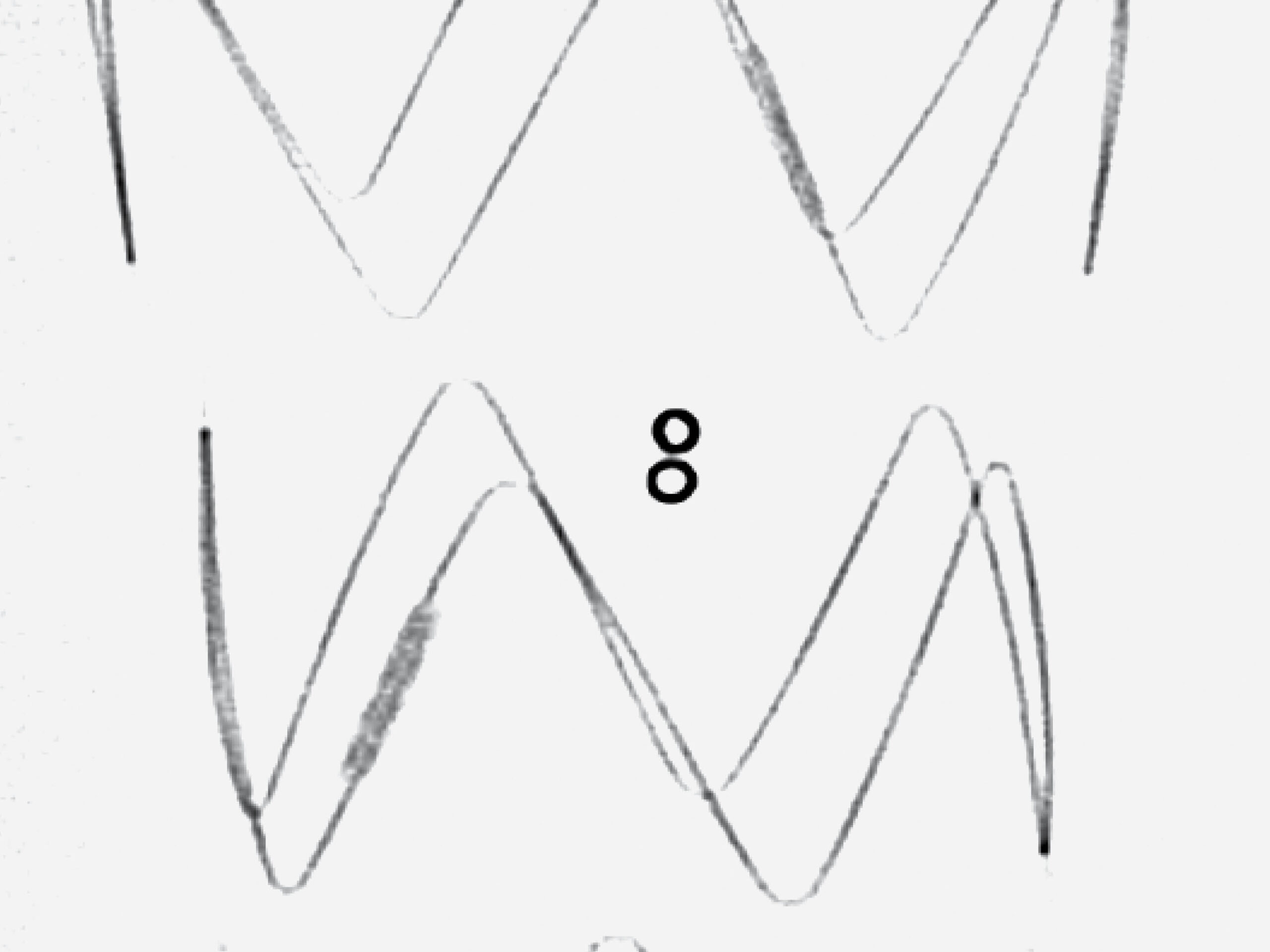
5. ARTIVEX MARKERS
For clear visibility, Artivex has platinum-iridium X-ray markers. Two vertical ring markers (double ring markers) indicate the Tapering.
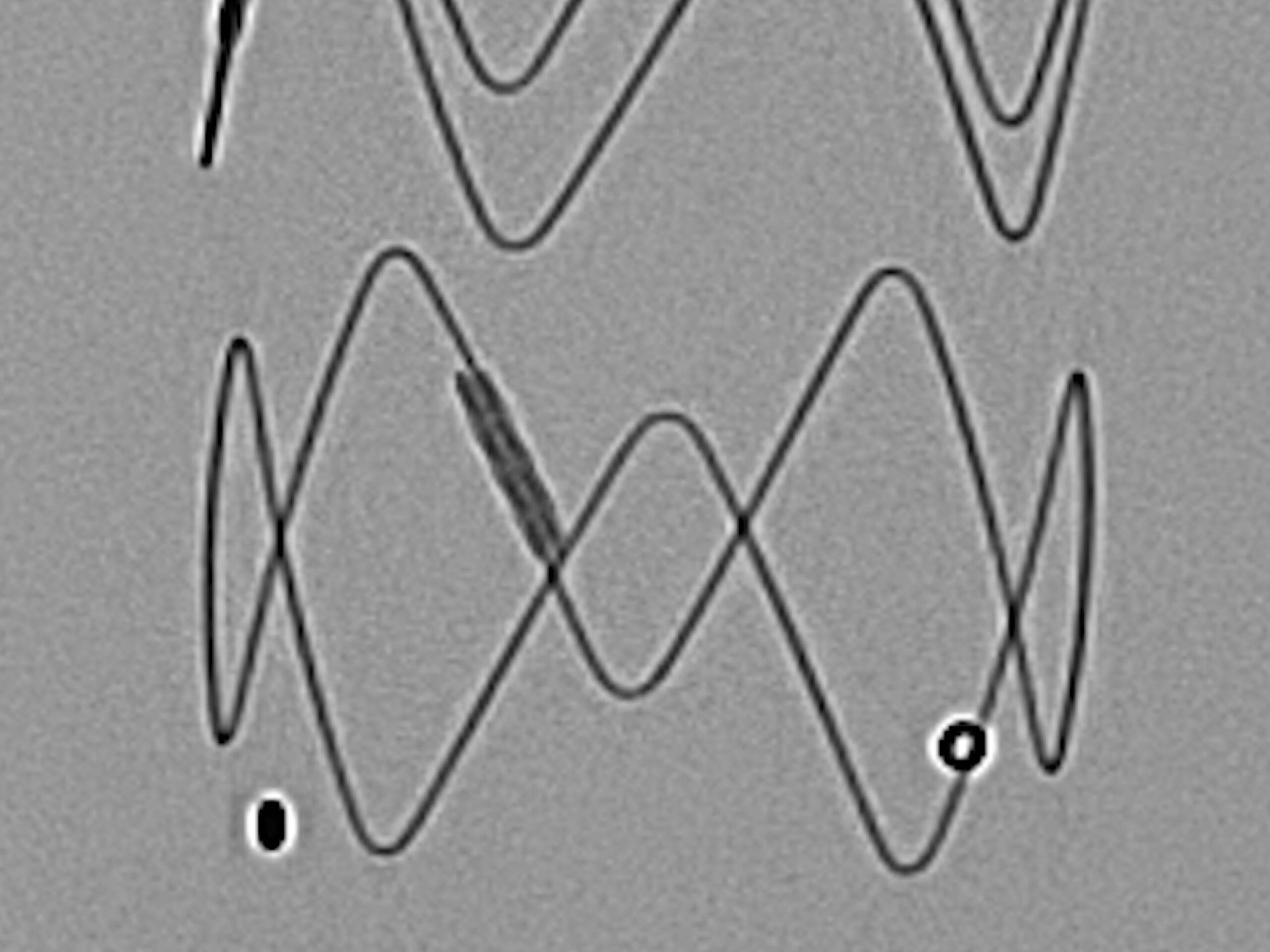
6. ARTIVEX MARKERS
For clear visibility, Artivex has platinum-iridium x-ray markers. Two ring markers define the distal end of the implant fabric.
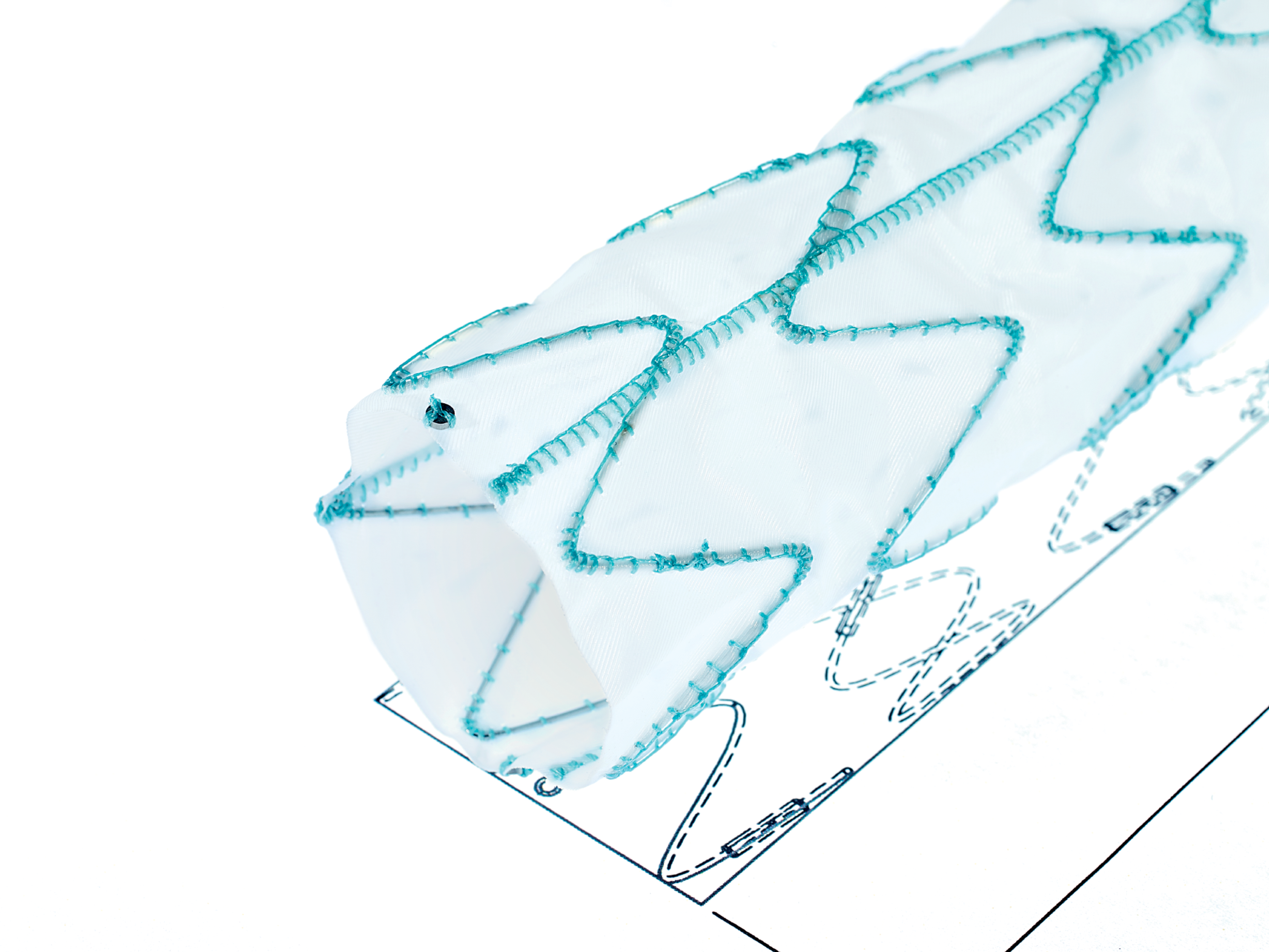
7. ARTIVEX DISTAL DESIGN
Straight Cut
1. FLEXIBLE CATHETER
(GRAFT COVER)
For accurate pushability and precise trackability.
2. RELEASE HANDLE
With squeeze-to-release mechanism.
3. CONTROL HANDLE
4. RELEASE BUTTON WITH RETAINING RING

Additional Resources
Explore additional resources for Artivex below. For further information or to contact a sales associate in your area, contact us.
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MWENG-0001.000. (2024-01)
| JOTEC GmbH, Lotzenäcker 23, 72379 Hechingen, Germany |

