
AMDS™
HYBRID PROSTHESIS
Elevate the Standard.
The world’s first device designed specifically to address the unique challenges of acute type A aortic dissection, specifically DeBakey Type I (ADTI)
.
Product Highlights
-
Reduction in Major Adverse Events (MAE’s)5
-
Prevention of DANE1,2,5
-
Resolution of Malperfusion1, 2
-
Promotes Positive Aortic Remodeling 1
-
Ease of Use1,2,4,5
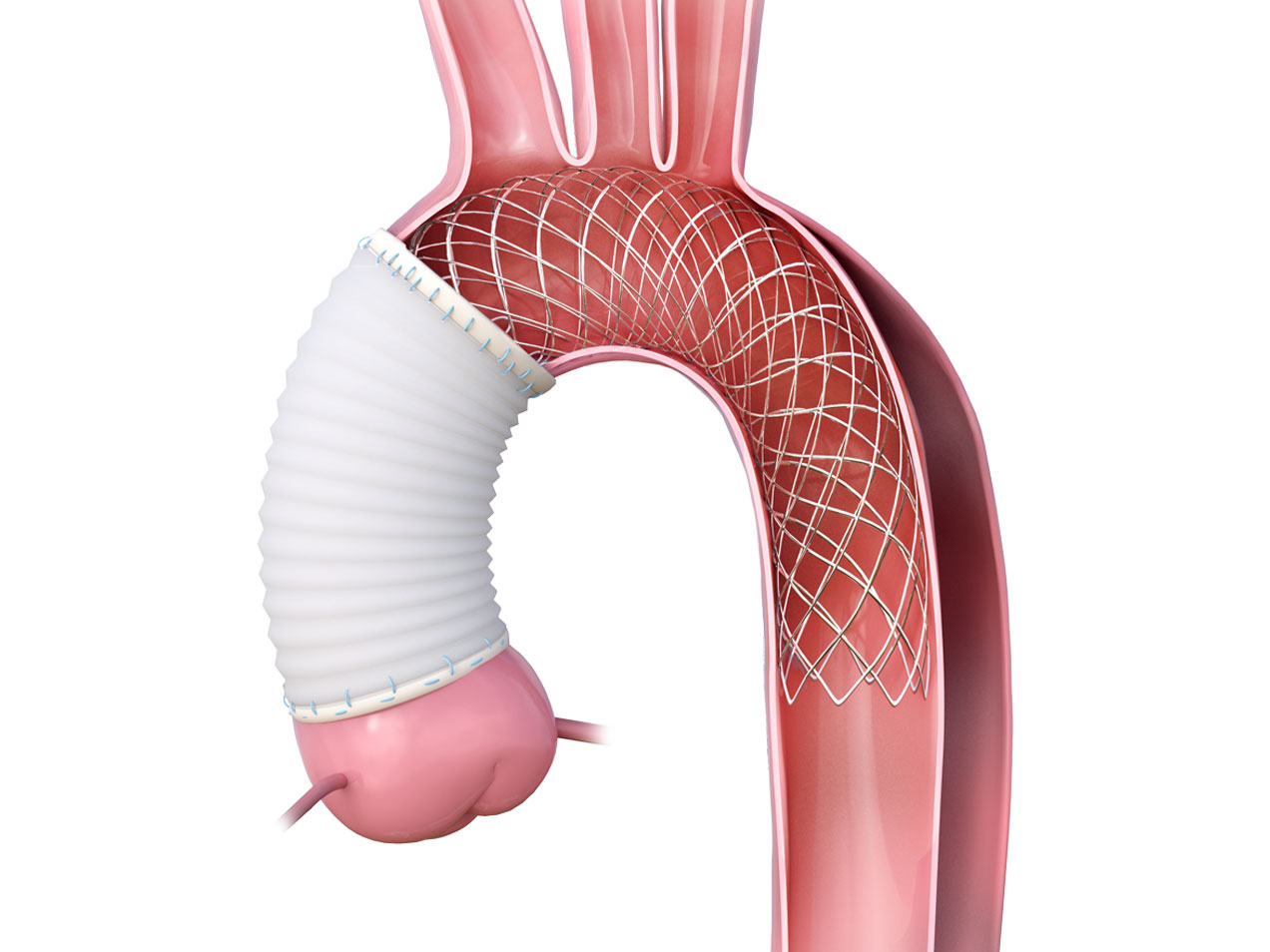
Product Overview
1. PTFE FELT CUFF

Component made of a PTFE felt tube is used to buttress and strengthen the aortic tissue in preparation to perform the conventional polyester graft to aorta anastomosis.
2. UNCOVERED NITINOL WIRE BRAIDED STENT
Expands and supports the true lumen across the aortic arch and descending aorta thereby stabilizing the aortic wall and promoting remodeling.
3. STENT SUPPORTED CUFF

The stent supported cuff of the AMDS and expansion of the AMDS from the arch distally, elevates and supports the intimal flap, reducing the tension on the suture line, avoiding the formation of DANEs in the friable anastomosis.
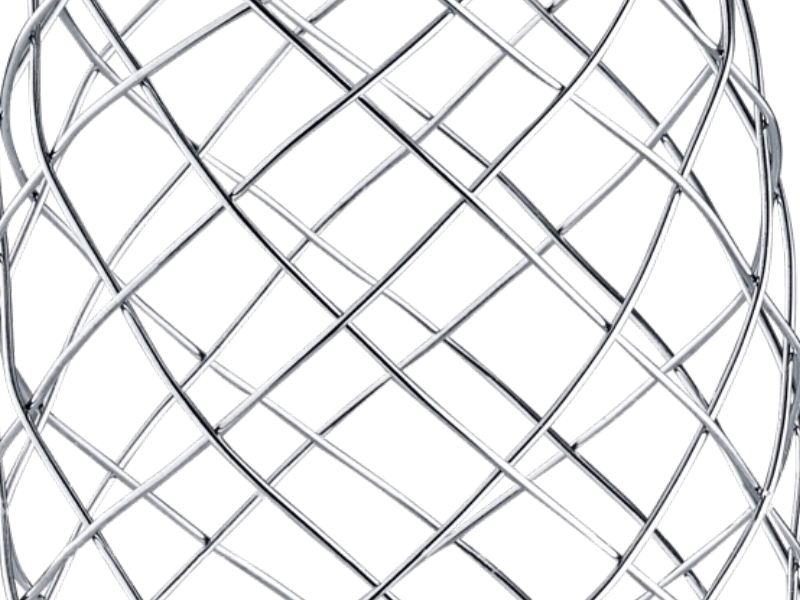
4. DESIGNED TO AVOID dSINE
When deployed in vessel, the distal ends of the stent are designed to point away from the aortic wall to avoid dSINEs.
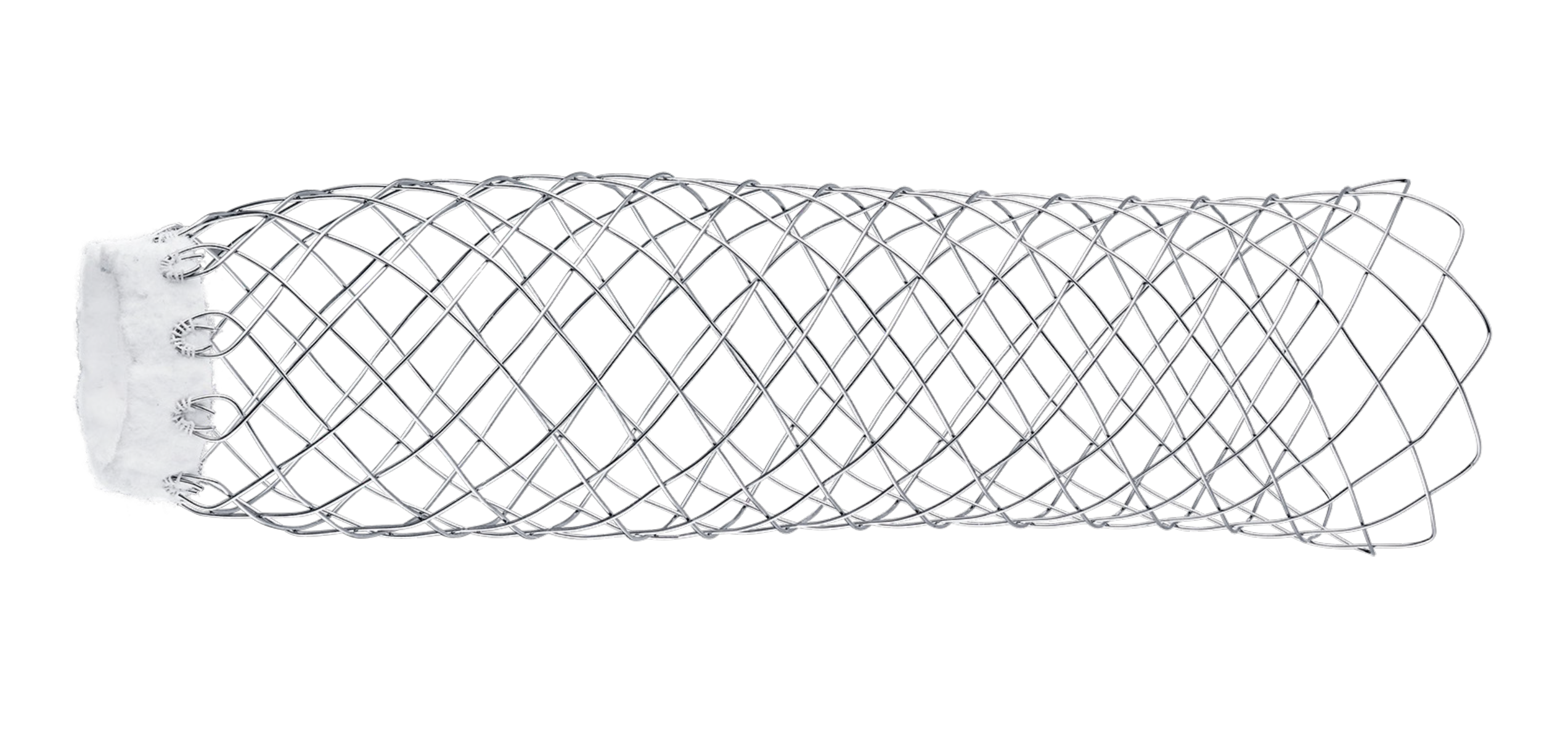
1. RED CAP
Not functional.

2. GUIDEWIRE (GW) EXIT
Compatible with 0.035” GW (optional).
3. HANDLE
Ergonomic handle for easy grip during delivery.

4. GREEN CAP
To deploy/release the suture constraining the stent.

5. PROTECTIVE SHEATH
For simple and atraumatic introduction to the transected aorta (6 cm long).

6. LOADED STENT
Flexible catheter shaft for simple tracking of system and atraumatic to vessel.

7. PIGTAIL SHAPED TIP
Pigtail tip reduces risk of device tracking into FL via a entry tear/fenestration.

“Knowing that you’re giving people surgery that is not having any additional complexity but with a better outcome is very reassuring.”
A new tool in the toolbox for surgeons to do a better operation at the time of an index operation for a DeBakey I.
Clinical Evidence
Proven Clinical Results
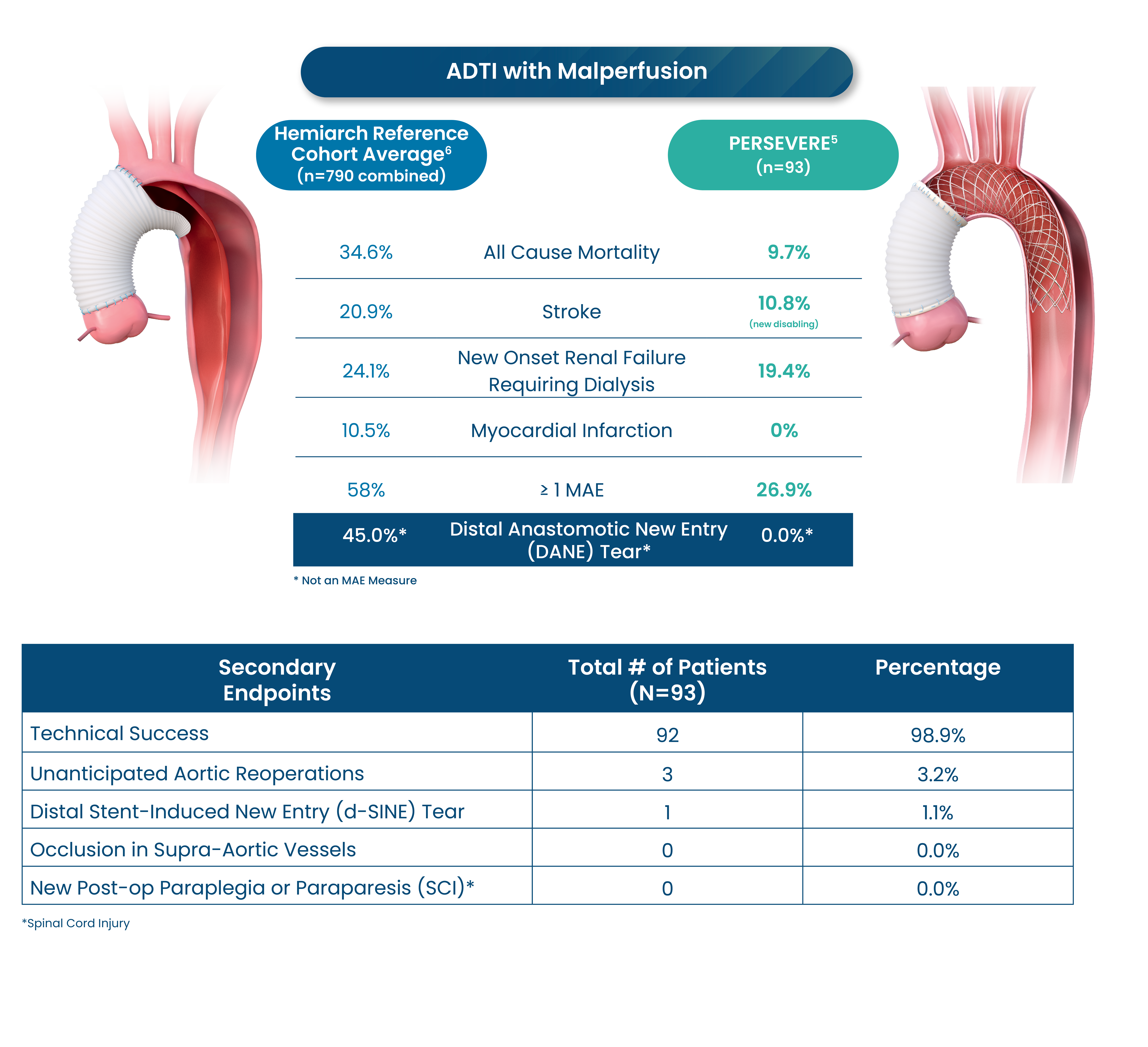
- The 30-day data demonstrates that the use of AMDS significantly reduces 30-day MAEs, including mortality, in the surgical treatment of ADTI patients complicated by malperfusion.5
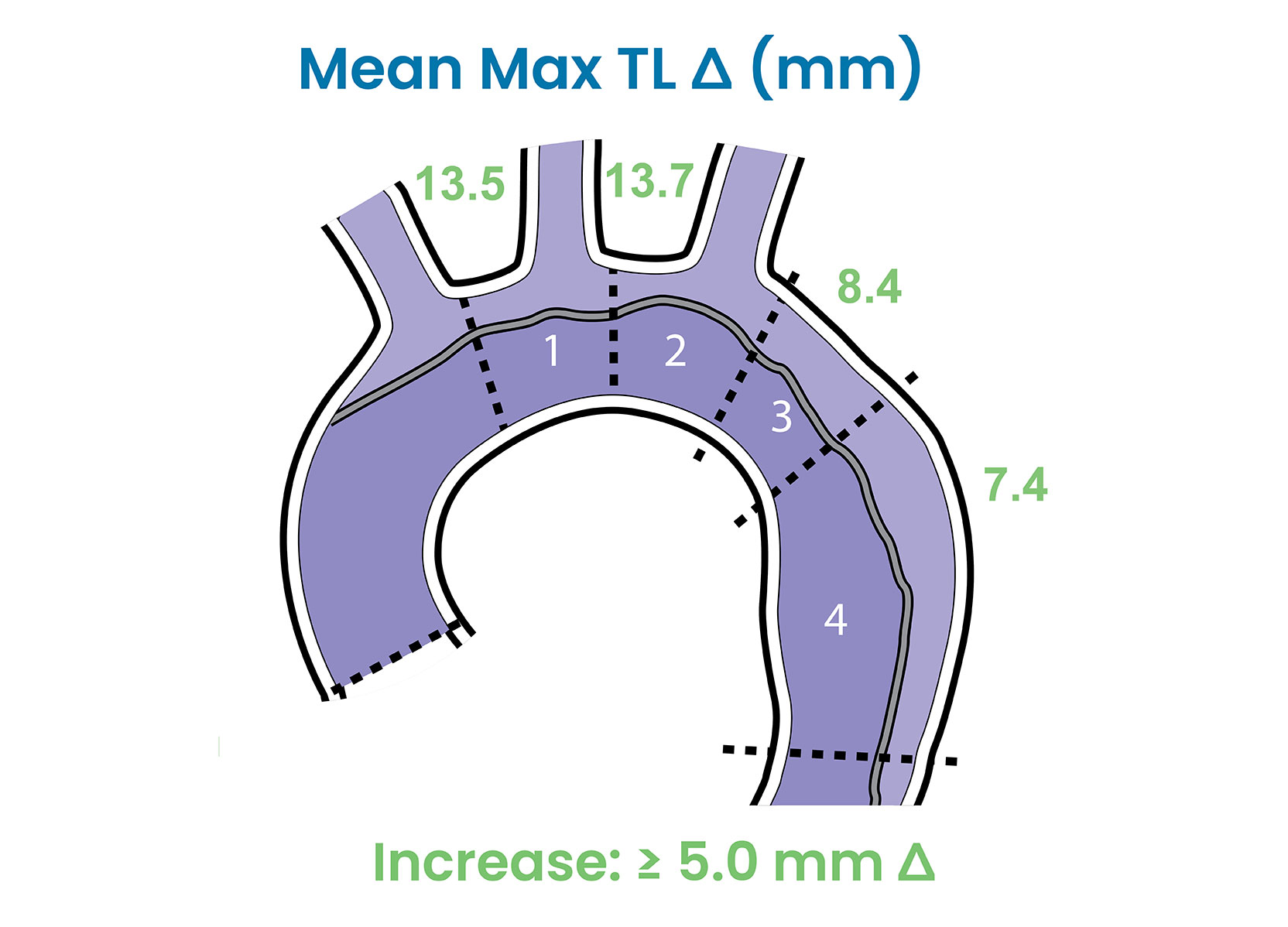
True Lumen Expansion
- DARTS study demonstrated sustained true lumen expansion ≥ 5.0 mm in aortic Zones 1-4 from pre-op to 3 years, post-op. 3
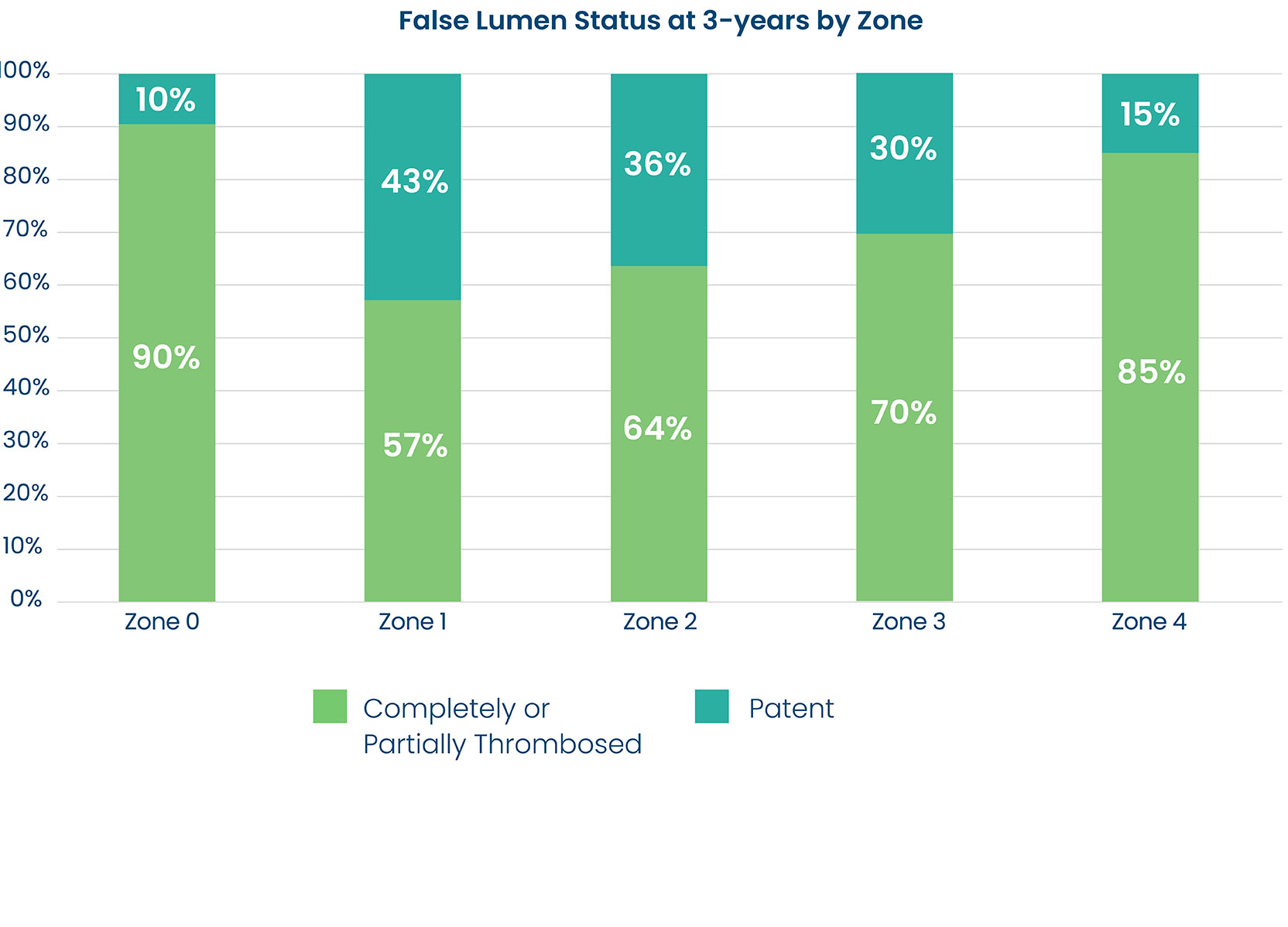
False Lumen Reduction
- DARTS study demonstrated complete or partial false lumen thrombosis in the majority of patients in Zones 0-4 at 3 years post-op.3
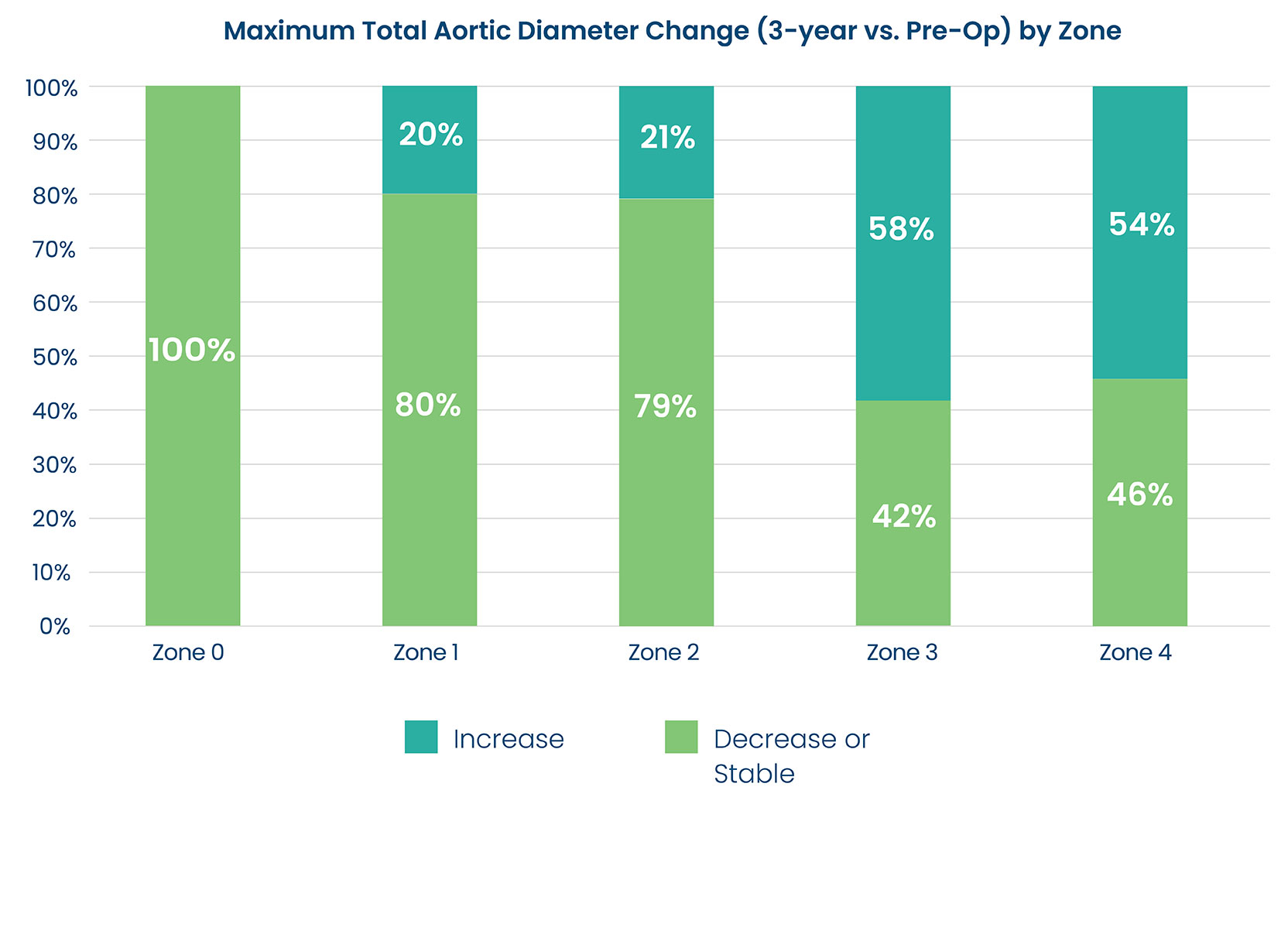
Total Aortic Diameter Stabilization
-
DARTS study demonstrated total aortic diameter stability or decrease in a majority of patients in Zones 0-2 and half of the patients in Zones 3 and 4 at 3 years, post-op.3
Additional Resources
Explore additional resources for AMDS below. For further information or to contact a sales associate in your area, contact us.
- Bozso, Sabin J., et al. “Three-year outcomes of the dissected aorta repair through stent implantation trial.” The Journal of Thoracic and Cardiovascular Surgery (2022).
- Bozso, Sabin J., et al. “Midterm outcomes of the dissected aorta repair through stent implantation trial.” The Annals of Thoracic Surgery 111.2 (2021): 463-470.
- DARTS Internal Data On File.
- Montagner, Matteo, et al. “The arch remodelling stent for DeBakey I acute aortic dissection: experience with 100 implantations.” European Journal of Cardio-Thoracic Surgery 62.2 (2022): ezac384.
-
Szeto WY, Fukuhara S, Fleischman F, Sultan I, Brinkman W, Arnaoutakis G, Takayama H, Eudailey K, Brinster D, Jassar A, DeRose J, Brown C, Farrington W, Moon MC. A novel hybrid prosthesis for open repair of acute DeBakey type I dissection with malperfusion: Early results from the PERSEVERE trial. J Thorac Cardiovasc Surg. 2024 Aug6:S0022-5223(24)00677-9.
- Zindovic I, 2019. Pacini D, 2013. Girdauskas E, 2009. Geirsson A, 2007. and Bossone E, 2002
| Ascyrus Medical GmbH, Grosse Gallusstrasse, 60312 Frankfurt, Germany |

