April 27 - 30, 2024
at the Metro Toronto Convention Center, Toronto, ON, Canada
Booth #605
Visit us at Booth #605 and check out the additional activities below.
Jump to a section to learn more:
AATS Late Breaking Science
This year, AATS has put together a top-notch scientific program. Be sure to check out some of the key sessions we found interesting. Remember that you must be registered with the annual meeting to attend any sessions.
Sunday, April 28th | 8:12 AM
Novel Aortic Arch Hybrid Prosthesis in Acute DeBakey Type I Dissection- Early Results of the PERSEVERE Study

Shinichi Fukuhara, MD
University of Michigan
Sunday, April 28th | 11:01 AM
Registry Results of Low-Dose Warfarin with a Novel Mechanical Aortic Valve Prosthesis: 5 Year Results

Marc Gerdisch, MD
Franciscan Health
Ongoing Clinical Trials
Stop by our booth to learn more.
PERSEVERE Pivotal IDE Study
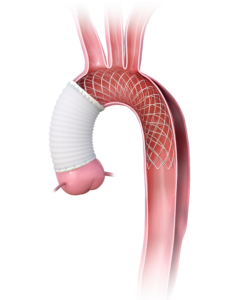 |
A ProspEctive, Single ARm, Multi-Center Clinical InveStigation to EValuatE the Safety and Effectiveness of AMDS* in the TREatment of Acute DeBakey Type I Dissection Pivotal IDE Study. AMDS is intended for aortic repair, aortic remodeling, and re-expansion of the intimal flap within the ascending aorta, aortic arch, and into the descending aorta for patients with acute DeBakey I aortic dissection undergoing open surgical repair within 0 to 14 days after diagnosis. |
Triomphe Pivotal IDE Study
 |
Prospective, non-randomized, multi-center clinical investigation of the NEXUS™ Aortic Arch Stent Graft System (NEXUS) for the treatment of thoracic aortic lesions involving the aortic arch with a proximal landing zone, native or previously implanted surgical graft, in the ascending aorta and with a brachiocephalic trunk native landing zone. |

